In this activity, learners use ethanoic anhydride to convert 2-hydroxybenzoic acid into aspirin
The reaction takes place easily in acidic solution, but the product is formed as part of a mixture containing several other compounds.
Equipment
Apparatus
- Access to a fume cupboard
- Pear shaped flask, 25 cm3
- Hot water bath
- Measuring cylinder, 10 cm3
- Bath of iced water
- Glass stirring rod
- Buchner funnel and suction
- Watch glass
Chemicals
- 2-Hydroxybenzoic acid, 1 g
- Ethanoic anhydride, 2 cm3
- Eight drops of concentrated phosphoric acid
Health, safety and technical notes
- Read our standard health and safety guidance here.
- Wear eye protection.
- Sulfuric acid can be used in place of phosphoric acid, but may give lower yields.
- Some teachers have reported problems which were due to using ethanoic anhydride that had already been hydrolised to ethanoic acid. Add a drop to water to ensure it is still reactive.
- If no precipitate appears, scratch the inside of the beaker with a glass rod or add a seed crystal of aspirin.
- As much as 40% of the mass of product after filtering may be water. Overnight drying is preferable to oven drying.
- Students should obtain about 0.9 g of crude product from 1.0 g of 2-hydroxybenzoic acid.
- For more information on hydroxybenzoic acid, see CLEAPSS Hazcard HC052
- For more information on ethanoic anhydride, see CLEAPSS Hazcard HC039
- For more information on phosphoric acid, see CLEAPSS Hazcard HC072
Procedure
Stage one
- Take about 1 g of your sample of 2-hydroxybenzoic acid and weigh it accurately.
- Put it into a dry pear shaped flask and add 2 cm3 of ethanoic anhydride followed by 8 drops of concentrated phosphoric acid.
- Put a condenser on the flask.
- In a fume cupboard, warm the mixture in a hot water bath, with swirling, until all the solid has dissolved and then warm for another 5 minutes.
Stage two
- Carefully add 5 cm3 of cold water to the solution.
- Stand the flask in a bath of iced water until precipitation appears to be complete. It may be necessary to stir vigorously with a glass rod to start the precipitation process.
- Filter off the product using a Buchner funnel and suction apparatus.
- Wash the product with a little cold water, transfer to a weighed watch glass and leave to dry overnight.
- Weigh your product.
Questions
- Formation of an iron-phenol compound with Fe3+ gives a definite colour. Does the crude product contain any phenol type impurities?
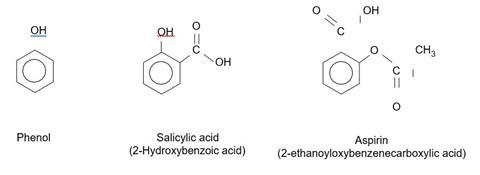
- Draw the structural formulae of phenol, 2-hydroxybenzoic acid and aspirin. Identify the functional group most likely to be reacting with the Fe3+ ions.
Answers
- The crude product may contain 2-hydroxybenzoic acid, as well as water or ethanoic acid as impurities. 2-Hydroxybenzoic acid can be formed either from incomplete reaction or from hydrolysis of the product during its isolation.
-
The OH group attached to the benzene ring produces a purple colour with Fe3+(aq) ions. The OH group in aspirin is part of the carboxylic acid group and does not react in the same way.
Downloads
The preparation of aspirin - teacher notes pdf
PDF, Size 0.2 mbThe preparation of aspirin - teacher notes
Word, Size 0.11 mbThe preparation of aspirin - student sheet pdf
PDF, Size 0.15 mbThe preparation of aspirin - student sheet
Word, Size 82.27 kb
Additional information
These resources were compiled by David Lewis and edited by Colin Osborne and Maria Pack.
Aspirin book
- 1
- 2
- 3
- 4
 Currently reading
Currently readingThe preparation of aspirin
- 5
- 6
- 7
- 8
- 9

























No comments yet