Learners can distil oil of wintergreen, and prepare salicylic acid, through the preparation of Gaultheriae procunbers leaves
This resource accompanies the article Fixing the funeral footprint which looks at sustainable ways to remember the dead. Water cremations use a similar technique of alkaline hydrolysis.
Learning objectives
- Use the following practical apparatus and techniques:
- Water bath or electric heater for heating.
- Quickfit® apparatus for heating and reflux.
- Filtration under reduced pressure.
- Safe handling of hazardous chemicals.
- Understand alkaline hydrolysis of esters.
- Calculate percentage yield.
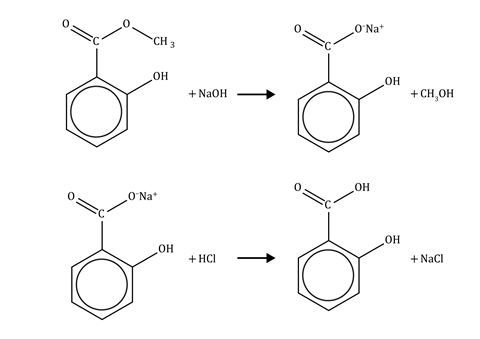
This activity is adapted from the second chapter of the book Aspirin and is suitable for 16–18 year-old learners studying the chemistry of carboxylic acids and esters. The reaction is an example of alkaline hydrolysis of an ester, followed by neutralisation of the salt with a strong acid to form the carboxylic acid (see equations below). The activity is also an opportunity for learners to develop and assess the experimental skills associated with the apparatus and techniques listed above.
Background
Many organic compounds are found in plants. 2-hydroxybenzoic acid (salicylic acid) can be made from methyl 2-hydroxybenzoate which is obtained as oil of wintergreen by distillation from the leaves of Gaultheria procunbers.
Oil of wintergreen is 98% methyl 2-hydroxybenzoate. This oil can be hydrolysed by boiling with aqueous sodium hydroxide for about 30 minutes. The reaction produces sodium 2-hydroxybenzoate, which can be converted into 2-hydroxybenzoic acid by adding hydrochloric acid.
Both oil of wintergreen (methyl 2-hydroxybenzoate) and salicylic acid (2-hydroxybenzoic acid) are widely used as pharmaceuticals. The manufacture of aspirin from salicylic acid is of major importance. Industrially, salicylic acid is manufactured at high temperature and pressure from the phenol sodium salt and carbon dioxide, with an annual worldwide production of about 50,000 tonnes.
The alkaline hydrolysis of esters is the basis of saponification (soap making) from natural oils and water cremation – a less environmentally harmful alternative to cremation by heat.
Apparatus
- 10 cm3 and 25 cm3 measuring cylinders (for the oil of wintergreen and sodium hydroxide)
- Weighing balance, ±0.01 g
- 50 cm3 pear-shaped flask fitted with a reflux condenser
Show learners how to correctly set up the Quickfit® apparatus with the water bath or electric heater and how to control the heat for steady reflux.
- Anti-bumping granules
- Water bath (eg 250 cm3 beaker) or an electric heater
- 100 cm3 beaker surrounded with ice and water in a larger container. (A larger plastic beaker is suitable to contain the ice.)
- Dropping pipette (to add the acid to the mixture)
- Stirring rod
- Spatula
- Buchner flask and suction apparatus
Show learners the filtration technique if they have not met it before.
- Watch glass
Chemicals
- Oil of wintergreen
- 2 mol dm–3 aqueous sodium hydroxide
- 2 mol dm–3 hydrochloric acid
- Several pieces of litmus or universal indicator paper on a white tile
- Ice-cold water. A wash bottle containing distilled water cooled in ice is suitable.
Safety equipment
- Splashproof goggles
- Chemical resistant gloves should be available for anyone who may have a cut or graze on their hands and for all if necessary.
Health, safety and technical notes
As with all experimental work in schools, you must carry out your own risk assessment in accordance with your employer’s health and safety policy. See also the RSC’s standard health and safety guidance.
The reactants and products in this experiment carry significant health and safety warnings. Pay particular attention to the hazard and disposal information in the technician notes.
- Sodium hydroxide (2 mol dm–3): DANGER can cause severe burns to the skin and is dangerous to the eyes, see CLEAPSS Hazcard HC091a.
- Oil of wintergreen: WARNING can cause skin irritation, serious eye irritation and may cause respiratory irritation, see CLEAPSS Hazcard HC052.
- Hydrochloric acid (2 mol dm–3): DANGER can cause severe skin and eye damage, may cause respiratory irritation, see CLEAPSS Hazcard HC047a.
- 2-hydroxybenzoic acid (salicylic acid) (product): DANGER harmful if swallowed, is suspected of damaging the unborn child, is harmful to aquatic life with long lasting effects and may cause an allergic skin reaction, see CLEAPSS Hazcard HC052.
- Methanol (product) DANGER highly flammable liquid and vaour and toxic if inhaled, swallowed or through skin contact, causes damage to organs, see See CLEAPPS Hazcard HC040b.
Procedure
Stage 1
Set up apparatus suitable for heating about 30 cm3 of reaction mixture using a water bath or an electric heater. Attach a condenser, as shown in the diagram, to prevent any volatile liquids escaping. Get your apparatus checked by your teacher before you start the reaction. Put on your splashproof goggles.
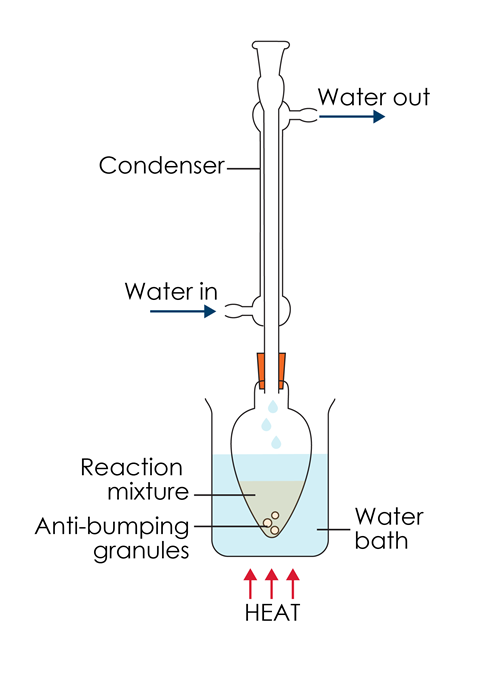
Weigh a small measuring cylinder containing approximately 1.7 cm3 of oil of wintergreen. Detach the condenser, add the oil to the flask and then reweigh the empty measuring cylinder and calculate the exact mass of oil of wintergreen added (which should be about 2 g). Add 25 cm3 of 2 mol dm–3 sodium hydroxide to the flask. Take care to avoid skin contact. Aqueous sodium hydroxide is also particularly prone to bumping, so you will need some anti-bumping granules. Reattach the condenser and check that the cooling water is on before heating gently under reflux for at least 30 minutes.
Switch off the heat and allow the mixture to cool. While the mixture cools, make a list of the possible compounds present in the mixture.
Stage 2
Pour the products into a small beaker surrounded by a mixture of ice and water. Add 2 mol dm–3 hydrochloric acid to the products dropwise, stirring all the time, until it is just acidic. Test for acidity by regularly touching the end of the stirring rod onto a piece of indicator paper. Why do you need to keep the mixture cool during this process?
Stage 3
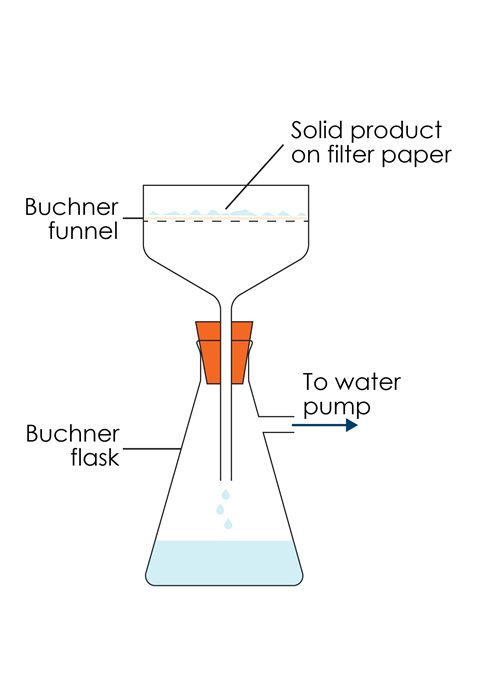
Filter the product using a Büchner funnel and suction apparatus. Wash the product with a little ice-cold water and transfer it to a weighed watch glass. Allow to dry overnight. Reweigh the watch glass containing the dried product.
Questions
Learners should include the answers to the following questions in their write up.
- How can you tell from observing the process in stage 1 that a new substance has been formed in the reaction?
- What has happened to the methanol formed in the reaction?
- Why do you need to keep the mixture cool while the acid is added in stage 2?
- What is oil of wintergreen used for nowadays?
- Calculate the mass of oil of wintergreen used and the mass of product formed, which will allow you to calculate the percentage yield of 2-hydroxybenzoic acid.
- Suggest a method you could use to further purify the product and then check the purity of the final product.
Answers
- There are two layers in the flask at the start of the preparation, but when the reaction is complete, the mixture is homogeneous (only has one layer).
- The methanol produced remains in the filtrate because it is completely miscible (capable of mixing) with water.
- The reaction of concentrated hydrochloric acid with the excess sodium hydroxide and the sodium salt of the salicylic acid is exothermic. The temperature also increases due to the concentrated acid dissolving. If the mixture warms up too much, more methanol evaporates, increasing the risk to health due to inhalation. Also, the salicylic acid becomes more soluble at higher temperatures, so the yield of solid will be lower.
- Oil of wintergreen is 98% methyl 2-hydroxybenzoate (also referred to as methyl salicylate), and it shows the medicinal properties of salicylates in general. It is not usually given by mouth but is readily absorbed by the skin. It is applied as a liniment for the relief of pain in lumbago, sciatica and rheumatic conditions, as well as for minor sports injuries.
- A good yield can be obtained in this experiment. 2.0 g of oil of wintergreen theoretically produces 1.7 g of dry 2-hydroxybenzoic acid.
- 2-hydroxybenzoic acid has a solubility in water of 2.5 g dm-3 at room temperature, which increases to 77.8 g dm-3 at 100°C, so recrystallisation would purify it without much loss of yield. The purity could be checked by measuring it has a sharp melting point of 159°C. A less pure sample would have a lower and less sharp melting temperature.
Further investigations
- Recrystallisation of the product could be carried out as above, and the melting point of the impure and purified product compared.
- The method could be used to investigate other examples of alkaline hydrolysis – eg saponification of natural oils.
- The atom economy and environmental impact of this preparation could be compared with the industrial manufacture of salicylic acid.
More resources
- Complete our Aspirin screen experiment, an interactive simulation of the synthesis of aspirin that features the same practical techniques used in the alkaline hydrolysis activity.
- Display posters about everyday compounds in your classroom, including the compound salicylic acid (also known as 2-hydroxybenzoic acid) which is prepared in this practical.
- Use this Exhibition Chemistry demonstration to show the effects of acid and alkali on living tissue and also the formation of soap from hydrolysed fat.
- Investigate the rate of hydrolysis of urea and assess your learners’ practical skills, including green chemistry questions on sustainable fertilisers.
- Meet Mike, a sustainability manager, who works with businesses and chemists to advise them on being more green through effective recycling and sustainable processes.
Downloads
The preparation of 2-hydroxybenzoic acid student sheet
Handout | PDF, Size 0.31 mbThe preparation of 2-hydroxybenzoic acid teacher notes and answers
Handout | PDF, Size 0.18 mbThe preparation of 2-hydroxybenzoic acid technician notes
Handout | PDF, Size 0.17 mbThe preparation of 2-hydroxybenzoic acid student sheet
Editable handout | Word, Size 0.6 mbThe preparation of 2-hydroxybenzoic acid teacher notes and answers
Editable handout | Word, Size 0.44 mbThe preparation of 2-hydroxybenzoic acid technician notes
Editable handout | Word, Size 0.16 mb
Additional information
These resources were compiled by David Lewis and edited by Colin Osborne and Maria Pack. This activity was updated in 2023 by Martin Bluemel.
Aspirin book
- 1
- 2
- 3
 Currently reading
Currently readingThe preparation of 2-hydroxybenzoic acid
- 4
- 5
- 6
- 7
- 8
- 9


























No comments yet