- Home
- I am a …
- Resources
- Collections
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- Literacy in science teaching
- More …
- Climate change and sustainability
- Alchemy
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Collections
- Education in Chemistry
- Teach Chemistry
- Events
- Teacher PD
- Enrichment
- Our work
- More navigation items
Analytical chemistry
Classroom resources featuring activities from our Analytical Chemistry professional development course for teachers
This collection is most valuable to those who have attended this course and wish to put into practice with their students some of the ideas and activities presented as part of that event. Please note that this list is not exhaustive; not all trainer activities have a corresponding classroom resource. In some circumstances there is variation between the training resource and classroom resource.
Titration screen experiment
Give students the opportunity to conduct their own titration experiment on a computer or tablet. This resource also includes a redox titration experiment.
Extracting iron from breakfast cereal
Try this class practical or demonstration to extract food-grade iron from breakfast cereals using neodymium magnets. Includes kit list and safety instructions.
Purifying an impure solid
Purify alum as an example of obtaining a pure chemical from an impure sample in this class practical. Includes kit list and safety instructions.
Chromatography of sweets | 11–14 years
Try this class practical to carry out chromatography using dye from different coloured M&M’s®. Includes kit list and safety instructions.
Detecting starch in food on a microscale
Test different foodstuffs for the presence of starch using iodine in this microscale class practical. Includes kit list and safety instructions.
Measuring the amount of vitamin C in fruit juices
Explore ascorbic acid in fruit juices through titration in this experiment, with specimen results and calculations, stock solutions, and detailed notes included.
ChemSpider - reviewed
How useful is ChemSpider database for school chemistry teachers?
Faces of Chemistry – Packaging gases
Discover how scientists from BOC remove gases from the air and use them in food packaging and processing.
Flame tests using metal salts
In this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts.
Chemical misconceptions II: Elements, compounds and mixtures
Explore and understand pure substances and mixtures; elements and compounds, through active study.
Separating sand and salt by filtering and evaporation
Try this class experiment to practise manipulating mixtures of soluble and insoluble materials by separating sand and salt. Includes kit list and safety instructions.
Chemical misconceptions II: Mass and dissolving
This exercise is primarily aimed at the 11–14 age range, to discover more about dissolving solids in liquids.
The fractional distillation of crude oil
Try this class practical or demonstration to simulate the industrial fractional distillation of crude oil. Includes kit list and safety instructions.
Leaf chromatography
Try this class practical to use paper chromatography to separate and investigate the pigments in a leaf. Includes kit list and safety instructions.
Chromatography worksheet
This activity extends the students’ understanding of chromatography. It links chromatography with particle theory and develops the tools of analogy and modelling.
Flame tests (the wooden splint method)
Find a new method to perform flame tests using wooden splints soaked in chlorides. Includes kit list and safety instructions.
Testing salts for anions and cations
A full range of chemicals will guide students into discovering how to identify the composition of unknown substances. Includes kit list and safry instructions.
Chromatography
This book teaches about modern chemical techniques without heavy emphasis on maths or physics. It includes descriptions of instruments and their applications.
Aspirin book
This book contains eight free-standing activities that can be used singly or as a coherent package in a wide range of teaching and learning situations for both academic and vocational courses.
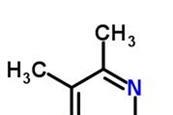
Finding the formula of hydrated copper(II) sulfate
In this experiment students will measure the mass of hydrated copper(II) sulfate before and after heating and use mole calculations to find the formula.
The determination of copper in brass
Try this microscale class practical to investigate how much copper there is in brass using nitric acid. Includes kit list and safety instructions.
Melting point determination
The measurement of melting points is a relatively straightforward procedure that is carried out to determine the purity of a compound or to assist with its identification. A pure compound will melt over a relatively narrow temperature range, impurities both lower and widen the temperature range over which a compound ...
Recovering water from copper(II) sulfate solution
Try this practical to introduce students to aqueous solutions by distilling water from copper(II) sulfate solution. Includes kit list and safety instructions.
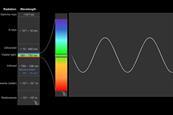
Introduction to spectroscopy
Get back to basics with this primer on the principles of spectroscopic techniques, including infrared (IR), ultraviolet-visible (UV-vis) and nuclear magnetic resonance (NMR). To make it even easier, each technique has clear explanations and descriptions supported by animations.
Spectroscopy introduction
Spectroscopy is the study of the way light (electromagnetic radiation) and matter interact. There are a number of different types of spectroscopic techniques and the basic principle shared by all is to shine a beam of a particular electromagnetic radiation on to a sample and observe how it responds to ...
IR student resources: Infrared spectroscopy
One of the first scientists to observe infrared radiation was William Herschel in the early 19th century. He noticed that when he attempted to record the temperature of each colour in visible light, the area just beyond red light gave a marked increase in temperature compared to the visible colours. ...
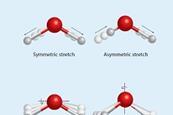
Mass spectrometry (MS)
Mass spectrometry is a powerful technique in the modern analytic laboratory. Learn the fundamental theory behind the operation of a mass spectrometer.
Gases from Air
An introduction to extracting gases from air.