Try this practical to introduce students to aqueous solutions using simple distillation
In this experiment, students use basic apparatus to evaporate and condense the water from copper(II) sulfate solution.
This is a simple introduction to aqueous solutions. Water is the solvent and it is only water that distils off when a solution is boiled. Other coloured solutions can be used – ink was the traditional one, but few students will still use ink pens and fewer will be aware of ‘bottles of ink’.
The apparatus may be quite complicated to set up for students at an early stage in their chemical careers. It is recommended that the flasks are set up with delivery tubes for the students. The clamped apparatus should also be set up in advance for the students if there are any doubts about their ability to do this correctly.
Pupils must be standing up while practical work is being carried out.
More water can be condensed if a beaker of water is held round the collecting tube. This leads to the idea of a water condenser as a more efficient way of collecting the water – see this procedure for demonstrating how to recover pure water from a solution using a water condenser.
The experiment will take about 20 minutes.
Equipment
Apparatus
- Eye protection
- Conical flask, 100 cm3
- Cork or bung with hole for delivery tube, to fit flask (see the diagram below)
- Lengths of glass tubing, bent as shown in the diagram
- Rubber connection tubing
- Stand and two clamps
- Tripod and gauze
- Bunsen burner
- Heat resistant mat
- Test tube
- Measuring cylinder, 25 cm3
Chemicals
- Copper(II) sulfate(VI) solution, about 0.5 M, 20 cm3 per group
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Copper(II) sulfate(VI) solution, CuSO4(aq) – see CLEAPSS Hazcard HC027c and Recipe Book RB031.
- The solution left in the flask should be recycled by diluting after the experiment (the exact concentration is not important, but note that solutions of greater concentration than 1 mol dm–3should be labelled HARMFUL).
Procedure
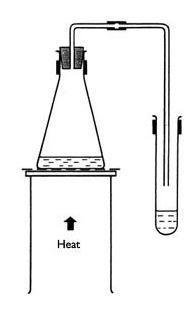
- Set up a Bunsen burner on the base of a stand placed on a heat resistant mat.
- Place a tripod and gauze above the burner.
- Clamp a flask and a test tube as shown in the diagram.
- Collect 20 cm3 of copper sulfate solution and place it in the flask.
- Fix the tubing in position as in the diagram.
- Light the Bunsen and heat the flask gently with a small flame. Do not heat to dryness.
- Water should distil over into the collecting tube.
Teaching notes
The colourless liquid collected can be assumed to be water at this stage. The main point is that it is not blue. It may be possible to detect a darkening of the original solution, showing that it is becoming more concentrated.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet