Ideas and resources to ensure learners understand structural and stereoisomerism
Imagine trying to give your friend a handshake, but you offer your right hand and they offer their left. It wouldn’t work. Similarly, a square peg doesn’t fit into a round hole.
The same is true for molecules. Each molecule has a unique shape that determines how it interacts with, for example, proteins in the body.
Take sight: isomerisation from 11-Z -retinal to all-E -retinal by a photon of light results in a change in the shape of the molecule from curved to straight (figure 1). This triggers a series of shape changes which ultimately generates a nerve impulse and allows you to see the light.
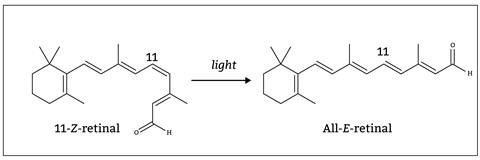
At 16–18, students learn about structural isomerism and stereoisomerism. They meet structural isomerism when learning about the homologous series and the rules of organic nomenclature. They go on to look at the three-dimensional shapes of molecules and meet stereoisomerism in both organic compounds and transition metal complexes.
What students need to know
Students need to know that:
- Isomers are compounds with the same molecular formula but with atoms and bonds arranged differently.
- Isomerism falls into two main categories – structural isomerism and stereoisomerism.
- Structural isomers have the same molecular formula but a different structural formula. They can be one of three types: position, functional group or chain isomers.
- Stereoisomers have the same molecular formula but differ in the arrangement of bonds in space. They can be one of two types: geometric or optical isomers.
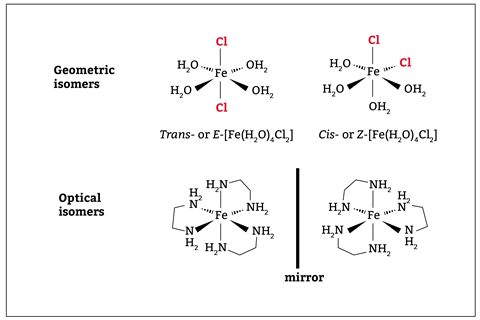
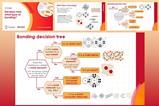
This handy infographic from Compound Interest summarises the different types of isomerism that students need to be familiar with in organic chemistry. With disubstituted transition metal complexes, both square planar and octahedral complexes exhibit geometric isomerism depending on the opposite (trans-) or adjacent (cis-) positioning of ligands. In octahedral complexes with two or more bidentate ligands or a multidentate ligand the complex may show optical isomerism if there is no plane of symmetry (figure 2).
Compound Interest’s infographic handily summarises the different types of isomerism that students need to be familiar with in organic chemistry (bit.ly/3YEvq3q). With transition metal complexes, both square planar and octahedral complexes exhibit geometric isomerism depending on the positioning of ligands. In octahedral complexes with two or more bidentate ligands or a multidentate ligand the complex may show optical isomerism if there is no plane of symmetry (figure 2).
Common misconceptions
In structural isomerism, students struggle to recognise when two molecules are the same, just drawn in a different orientation. Ask students to draw and name as many structural isomers as they can with the formula C6 H14. Then identify which are, in fact, the same molecule. This encourages students to focus on atom connectivity as opposed to the molecule’s overall shape.
Drawing functional group isomers also presents a significant challenge. Overcome this by introducing the concept early on then revisiting it as students meet the different homologous series.
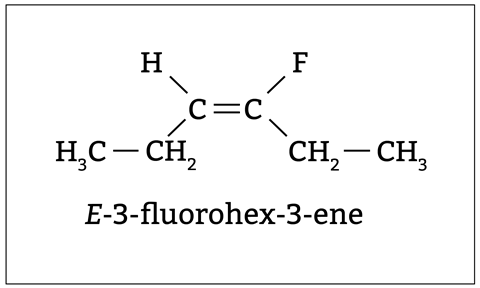
To know if alkenes are E - or Z -, students must identify the higher priority group attached to each carbon atom of the double bond. A common misconception is for students to assign priority by the atomic mass of attached groups. Instead, they should assign priority based on the atomic number of the atom directly attached to the carbon atom (see figure 3).
Ideas for your classroom
Encourage students to build models of molecules and manipulate them; rotate them and spin bonds. Build simple straight chain alkanes to demonstrate free rotation around C–C bonds and introduce the possibility of branching to explain chain isomerism. Progress to the incorporation of functional groups on the chain allowing for position isomerism. When introducing E/Z isomerism, build a model of a simple alkene to see the lack of rotation around the C=C bond. When students meet optical isomerism, make mirror images of molecules and see if they superimpose.

Download this
Investigating isomerism, for age range 16–18
Explore cis-trans isomerism in transition metal complexes with this experiment, including pre- and post-lab questions.
Resource includes:
Download this
Investigating cis-trans isomerism, for age range 16–18
Explore isomerism in transition metal complexes with this experiment, including pre- and post-lab questions.
Download the resource from the Education in Chemistry website: rsc.li/3DZkzcE

Students sometimes struggle to recognise the isomerism in octahedral transition metal complexes. Octahedral complexes, especially those with bi- and tridentate ligands, are difficult to draw in three dimensions. This means it is hard to recognise planes of symmetry and identify if structures are superimposable. Simplified models (figure 4) where you remove details on an atomic level can help students visualise the spiral nature of a complex.
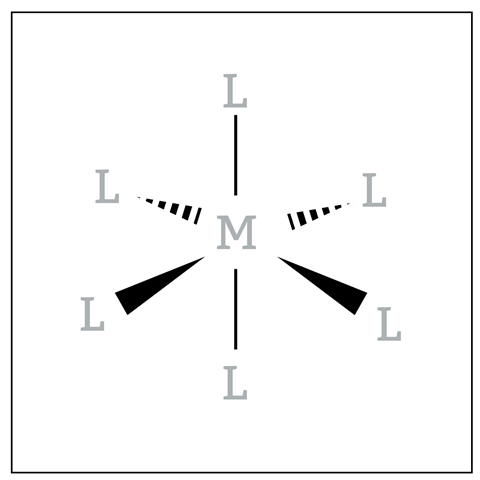
If students struggle with drawing in three dimensions, provide them with a blank template such as figure 5. With the same blank template on PowerPoint slides you can live draw ligands to focus students’ attention on the key aspect, namely the positioning of the ligands.
More resources
- Assess student understanding of geometric isomerism and introduce them to the role it in plays in the synthesis of molecular motors, with this article and accompanying resource.
- Use this simple demonstration to explore two enantiomers of limonene to discuss properties of stereoisomers.
- Share the story of Edith Humphrey, the first of Alfred Werner’s students to synthesise geometric isomers of octahedral complexes, with your students.
The three-dimensional nature of molecules and complexes is key to their biological activity. To add context students can research the thalidomide story or why Olympic skier Alain Baxter tested positive for methamphetamine. The Molecule of the month webpages provide an excellent starting point for student research at post-16.
The three-dimensional nature of molecules and complexes is key to their biological activity. To add context students can research the thalidomide story or why Olympic skier Alain Baxter tested positive for methamphetamine. The Molecule of the month website provides an excellent starting point for student research at post-16 (bit.ly/4hpFPHC).
More resources
- Assess student understanding of geometric isomerism and introduce them to the role it in plays in the synthesis of molecular motors, with the article, ‘Molecular motors power on’ and the accompanying resource: rsc.li/4ec19gR
- Use this simple demonstration using two enantiomers of limonene to discuss properties of stereoisomers with your students: rsc.li/3WoRcH4
- Share with your learners the story of Edith Humphrey, the first of Alfred Werner’s students to synthesise geometric isomers of octahedral complexes: rsc.li/3NKgOcx
Checking for understanding
Quickly assess students’ understanding with simple quiz questions that ask them to identify if a molecule demonstrates isomerism or not. Increase the challenge with questions, such as those in the assessment exercise in this CPD article or by using more complex structures such as phenolphthalein or ibuprofen.
Quickly assess students’ understanding with simple quiz questions that ask them to identify if a molecule demonstrates isomerism or not. Increase the challenge with questions, such as those in the assessment exercise in the CPD article, ‘How to introduce organic chemistry to your post-16 students’ (rsc.li/3YuuBZE) or by using more complex structures such as phenolphthalein or ibuprofen.
Take-home points
- Isomerism exists in both organic and inorganic complexes.
- Build in opportunities to revisit the different types of structural isomerism as student confidence in recognising functional groups and applying the rules of nomenclature increases.
- To be able to identify different stereoisomers, students need a good understanding of the three-dimensional nature of molecules and complexes, including which bonds they can and cannot rotate around.
- Put isomerism in context to inspire students to read beyond the curriculum and consider a career in chemistry.
Article and resource by Catherine Smith, chemistry lead at The Hinckley School in Leicestershire













1 Reader's comment