Get started
Watch a demonstration of this experiment and download the technician notes from the Education in Chemistry website: rsc.li/4k3jXCb
Learners come to our classrooms with a variety of pre-existing beliefs about evaporation, boiling and condensation that can inhibit further learning. Indeed, even teachers can have confused ideas. In a study on student teachers in Turkey, many had incorrect mental models of vapour pressure and more than half provided answers that revealed they were using a static model for a liquid–gas system, which did not reflect the dynamic equilibrium at play. The following demonstration is an excellent tool for probing the faulty models our students (and even we teachers) might be using.
Learners come to our classrooms with a variety of pre-existing beliefs about evaporation, boiling and condensation that can inhibit further learning. Indeed, even teachers can have confused ideas. In a study on student teachers in Turkey, many had incorrect mental models of vapour pressure and more than half provided answers that revealed they were using a static model for a liquid–gas system, which did not reflect the dynamic equilibrium at play (rsc.li/3TPjykk). The following pair of demonstrations are excellent tools for probing the faulty models our students (and even we teachers) might be using.
Use this demonstration when teaching changes of state at 11–14 and vaporisation at 16–18.
Kit
- 60 cm3 plastic syringe
- Syringe cap (or short piece of silicone tubing with a Hoffman clip)
- Anti-bumping granules
- Thermometer
- Kettle full of water
Health and safety
- Read our standard health and safety guidance and carry out a risk assessment before running a live demonstration.
- Take care when pouring hot water to avoid spilling it on yourself.
Health and safety
- Read our standard health and safety guidance and carry out a risk assessment before running a live demonstration (rsc.li/45YUM4h)
- Take care when pouring hot water to avoid spilling it on yourself.
Preparation
Remove the plunger from the syringe. Add a few anti-bumping granules and replace the plunger. Heat water in the kettle to 70–80°C. Water closer to boiling point can weaken the tip of the syringe and presents an unnecessary scalding risk, plus the purpose of the demonstration is to show water boiling far from 100°C. Below 70°C, the effect is too subtle to be obvious.
In front of the class
Pour some hot water into a beaker and show the temperature to the class. Ask them if the water is boiling and then if it is evaporating. Most 11–14 year-olds will agree it is not boiling and if you ask them to elaborate, they will say that there are no bubbles. Ask them what you could do to the water to make it boil. Most will suggest (perhaps pointing towards the kettle) that you will need to heat the water. Few will suggest an alternative method.
Turn the kettle on and ask the students to listen. Ask them what they hear. When they can hear bubbling, ask them to consider what is in those bubbles. Many students will claim that the bubbles are full of air. Explain there is another way of making water boil that doesn’t require heating.
Carefully draw liquid into the syringe until it is about one third full. Invert it and carefully depress the plunger until you have expelled all the air and seal with a syringe cap. Ask the students if there is any air left in the syringe to draw their attention to its absence and finally, holding the syringe vertically, pull back the plunger. Bubbles will stream from the anti-bumping granules.
Remind the class that there is no air in the syringe and ask them what must be in the bubbles. More students will now suggest the correct answer: gaseous water. Ask them again: was the liquid evaporating, was it boiling and did we heat it? Finally, help them to establish that boiling is evaporation with bubbles.
Teaching goal for 11–14
I was inspired to make the sequence above by work demonstrating how functional examples can help students move past misconceptions about evaporating and boiling. A Korean study asked children aged 9–12 about situations associated with boiling and evaporation; 68% (of 11–12 year-olds) misidentified situations where a liquid was heated as boiling and 71% misclassified boiling scenarios where no heating took place as evaporation.
I was inspired to make the sequence above by work demonstrating how functional examples can help students move past misconceptions about evaporating and boiling. A Korean study asked children aged 9–12 about situations associated with boiling and evaporation; 68% (of 11–12 year-olds) misidentified situations where a liquid was heated as boiling and 71% misclassified boiling scenarios where no heating took place as evaporation (rsc.li/3P5szeg).
Even without a submicroscopic model of boiling, learners could understand the difference after the researchers showed water boiling under reduced pressure. 11–14 year-olds need to understand that the vapour in the bubbles needs to push back against the pressure from the atmosphere above, otherwise the bubbles will collapse. Point out to them that the louder, higher-pitched sound of the bubbles when the kettle was heating was the sound of these tiny bubbles collapsing. Only when the vapour pressure approaches atmospheric pressure is the bubble able to maintain its size then grow. This causes the drop in pitch as the kettle approaches the boil.
Teaching goal for 16–18
Vapour pressure comes and goes from 16–18 curriculums – it’s not in the most recent International baccalaureate diploma programme specification and does not currently appear in A-level specifications. However, some A-level specifications require students to solve problems involving partial pressure. For this reason, you can use the above demonstration to reinforce the role of vapour pressure in boiling before you test learners’ understanding of vapour pressure in a syringe with air.
As shown in the Turkish investigation, faulty mental models about vaporisation can persist into adulthood. When researchers asked student teachers to consider the situation in figure 1, 59% gave responses which indicated that they had fixed mental models of vapour pressure (or for A-level teachers, the partial pressure of the water).
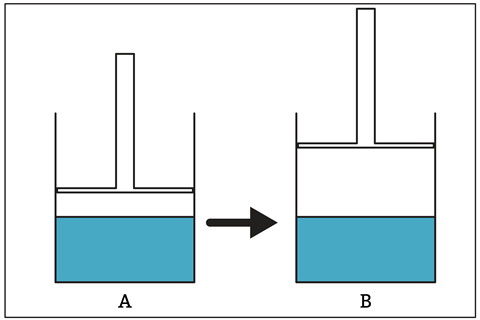
The researchers identified the most common (and incorrect) response as the vapour pressure in A being greater than in B because the vapour volume increases in container B and (by the ideal gas law) pressure and volume are inversely proportional.
As shown in the Turkish investigation, faulty mental models about vaporisation can persist into adulthood. When researchers asked student teachers to compare the vapour pressure in two piston-cylinder devices, 59% gave responses which indicated that they had fixed mental models of vapour pressure (or for A-level teachers, the partial pressure of the water).
The study summarised the most common (and incorrect) response as the vapour pressure with the piston pushed down being greater than in the cylinder with piston pulled up, because the vapour volume increases in the latter container and (by the ideal gas law) pressure and volume are inversely proportional.
The student teachers’ answers suggested they did not consider that the number of vapour particles could and would change as the dynamic equilibrium between the liquid and the vapour shifted. The vapour pressure of a particular liquid depends only on temperature.
You can test this faulty model further with the Cloud in a bottle demonstration, which impresses all ages.
You can test this faulty model further with the ‘Cloud in a bottle’ demonstration on page 40, which impresses all ages.
Declan Fleming
Downloads
Boiling without heating technician notes
Handout | PDF, Size 0.18 mbBoiling without heating technician notes
Editable handout | Word, Size 0.49 mb
Additional information
Thank you to Pavel (Pasha) Mikhalkov for co-presenting this Exhibition chemistry video.
















































No comments yet