Spur your students on with this step-by-step approach to covalent bonding
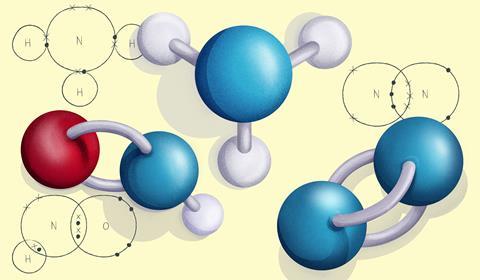
Once they have mastered electron configuration diagrams, show your learners how they can adapt them to show structure and bonding in covalent and ionic compounds. These structural diagrams depict only the outer, or valence, shell electrons and are known as dot and cross diagrams. Electrons from different atoms use alternating symbols, usually a dot and a cross, to show which atom the electrons have come from.
Let’s take a closer look at some compounds containing nitrogen. Remember that nitrogen has five electrons in its outer shell.
How to draw a dot and cross diagram for ammonia
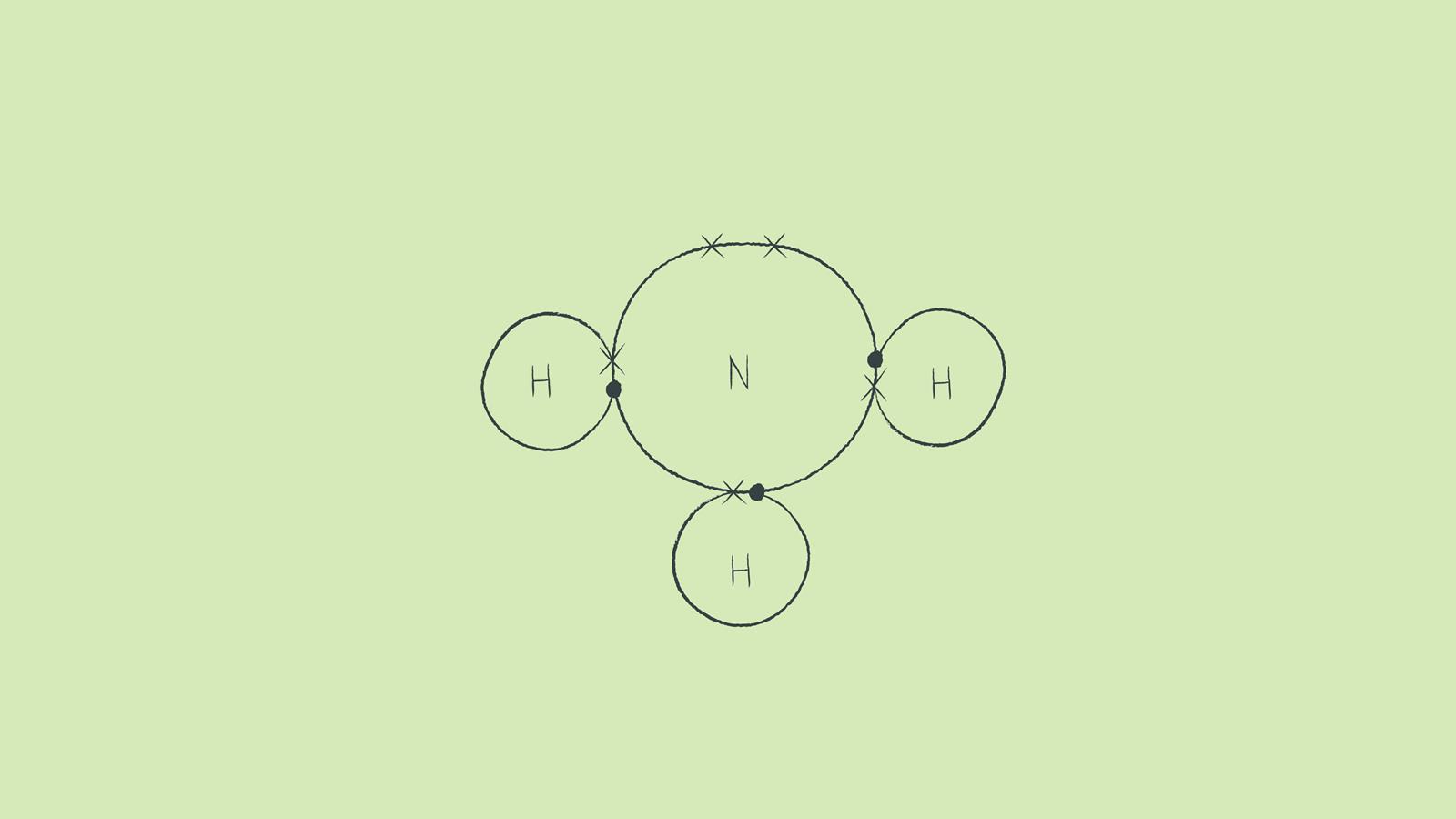
In NH3, commonly known as ammonia, nitrogen forms three single covalent bonds with three hydrogen atoms.
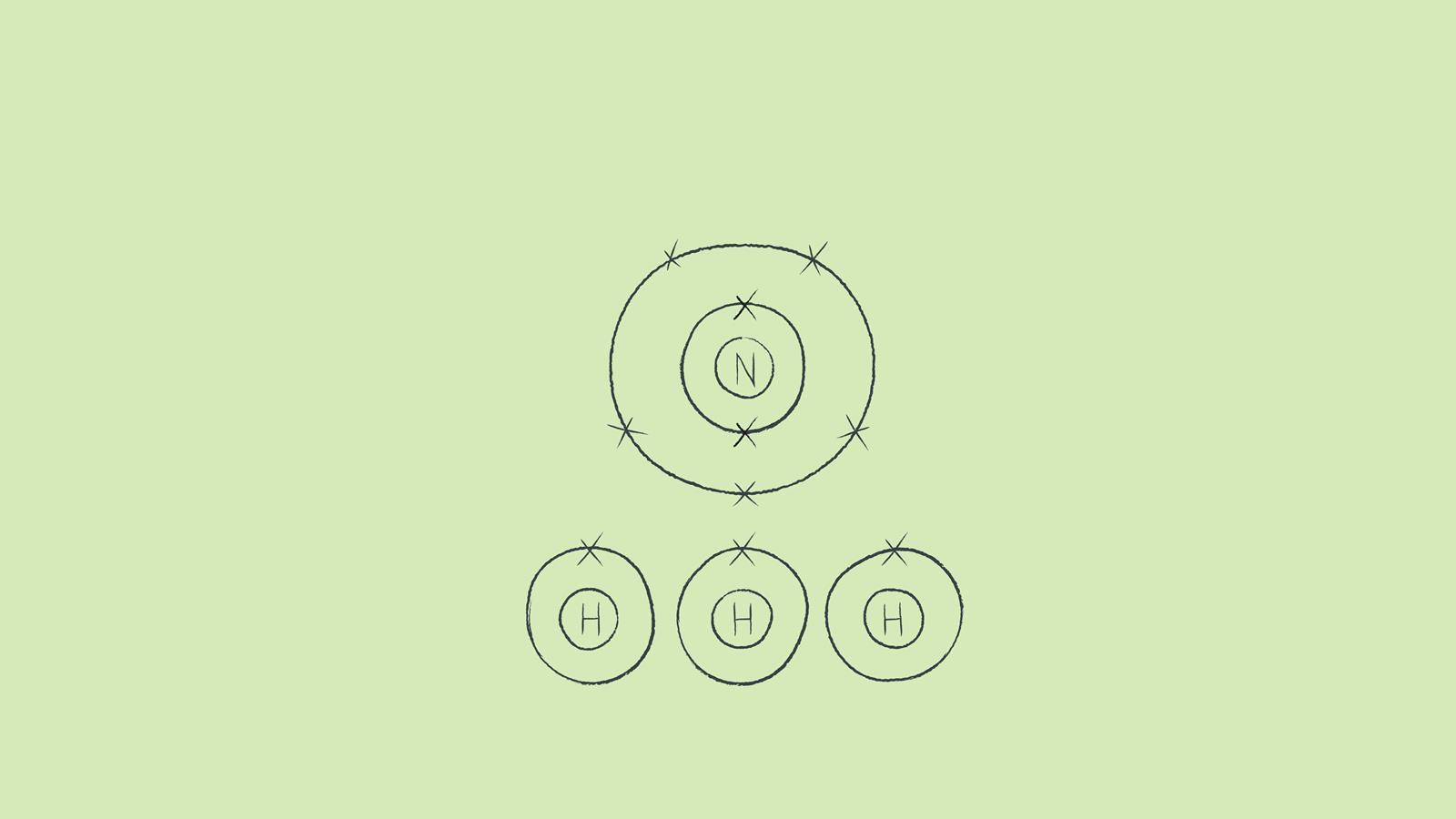
While you are learning how to draw dot and cross diagrams it’s useful to start with something you are already familiar with: electron configuration diagrams. Sketch out the electron configuration diagrams for each of the atoms.
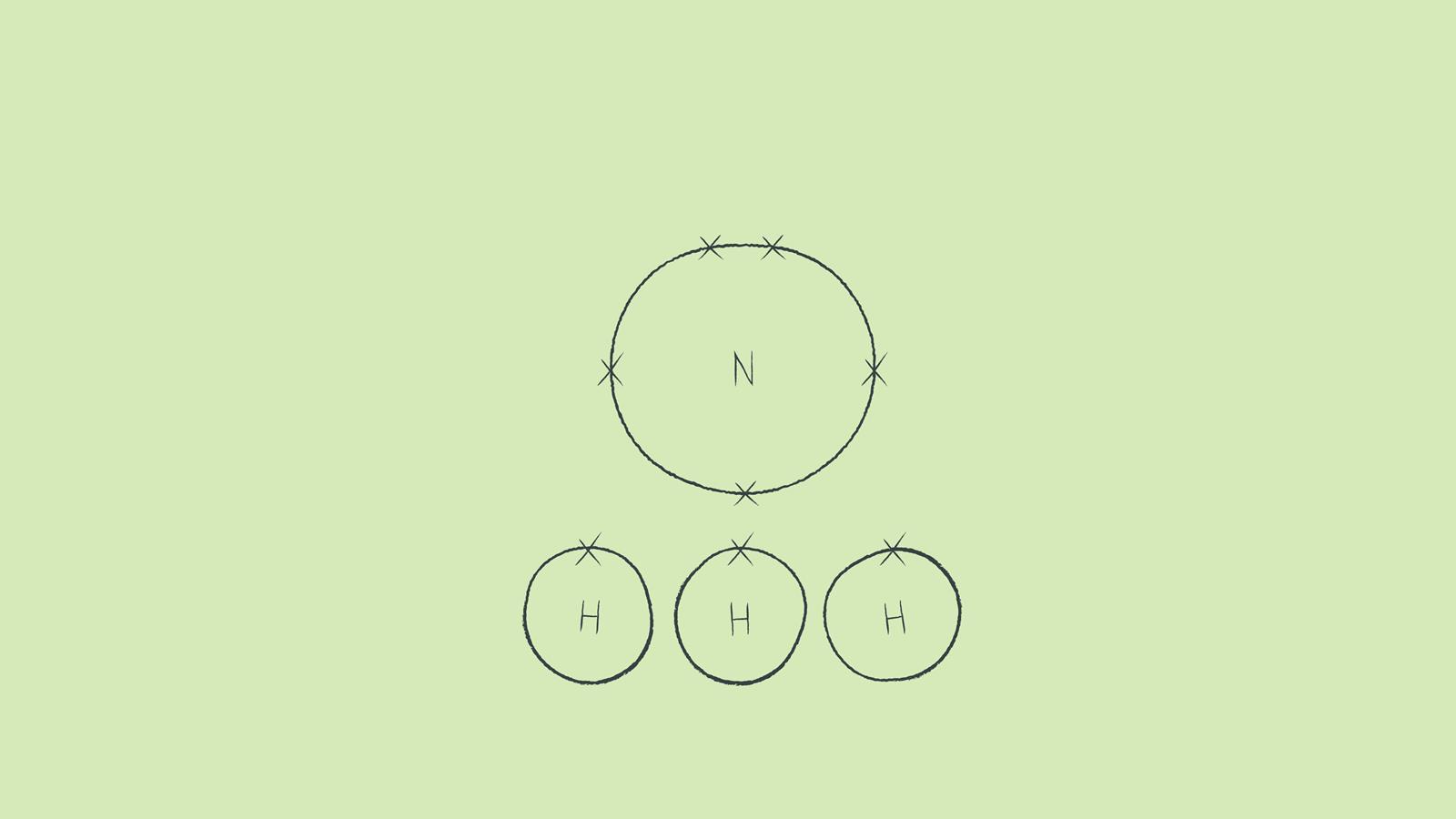
Draw the outer shell of each atom. It’s a good idea to draw the electrons in pairs but remember to use a pencil as you may have to move the electrons around to fit the structure. You don't need to put a circle around the symbol for the nucleus.
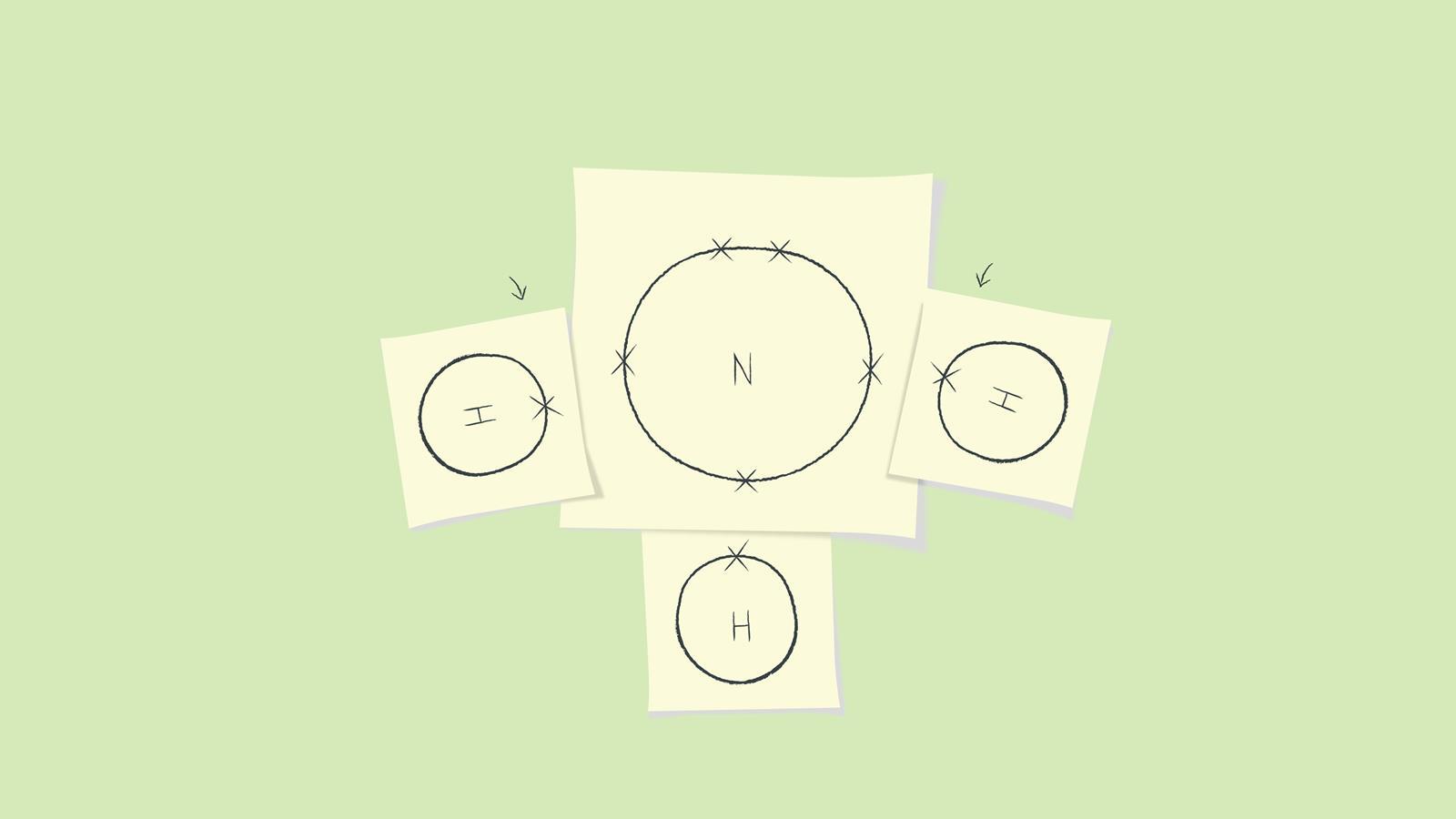
Arrange the atoms so that the valence electrons will complete the outer shells of all the atoms in a simple covalent molecule. While you are learning, draw each atom on a small piece of paper so you can easily rearrange them.
Draw the completed dot and cross diagram. Here we have changed the electrons on the hydrogen atoms to be dots. You can alternate the dots and crosses in simple diagrams or use other colours or symbols for larger diagrams. This dot and cross diagram shows the outer shells touching.
This method is easiest for single covalent bonds as there is not much room where the atoms’ shells touch for drawing lots of electron pairs.
-

-

Covalent bonding tiles worksheet
Use the accompanying fact sheet and worksheet to get your students practising dot and cross diagrams. Go to the accompanying resource for further information about how to use the Covalent bonding tiles.
View and download more infographics
How to draw double and triple bonds
This first method (blue background) is useful for double and triple bonds as it gives more space for drawing electrons. This second method (pink background) is useful for checking that you have drawn the right number of electrons in the outer shell of each atom.
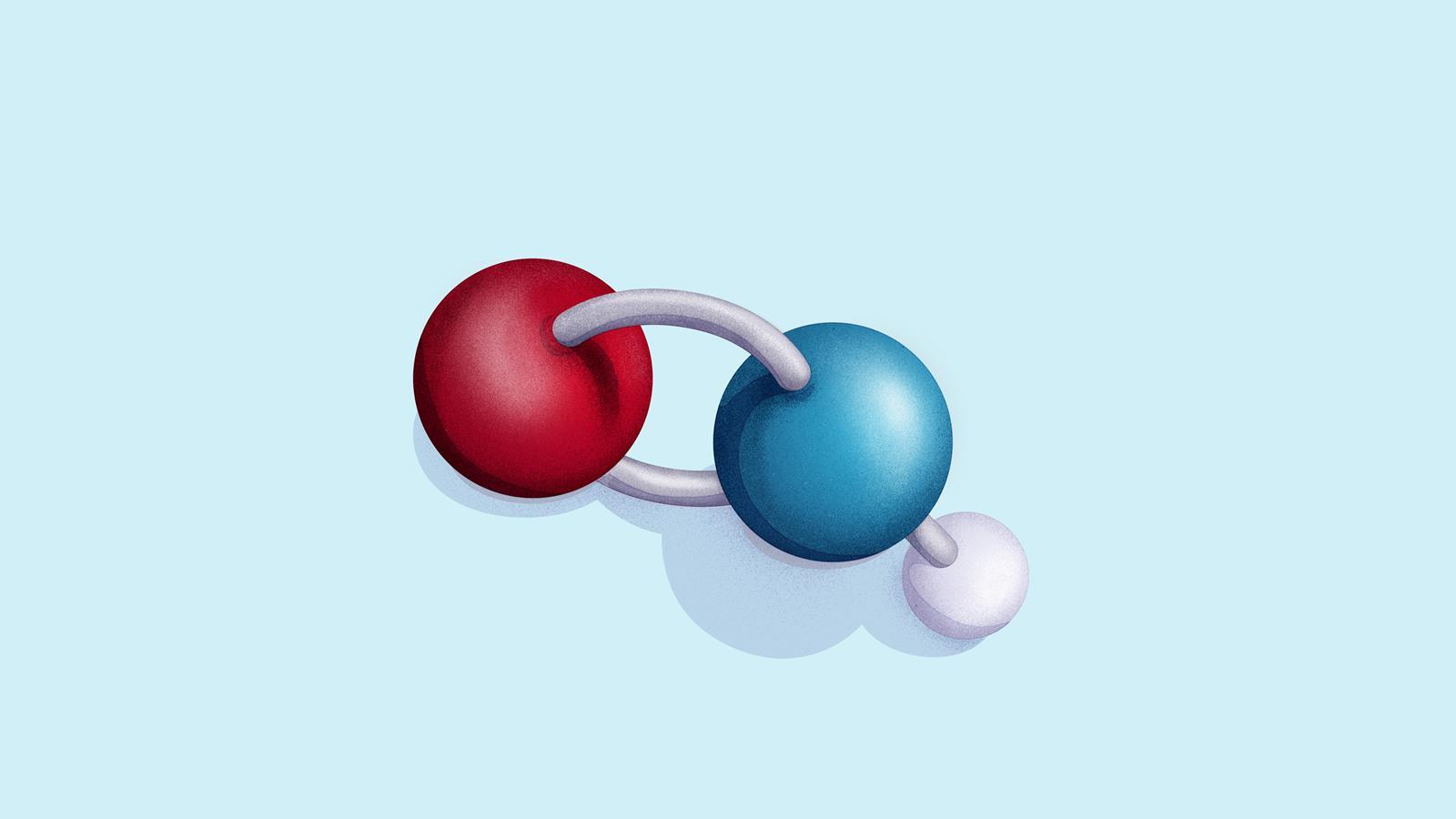
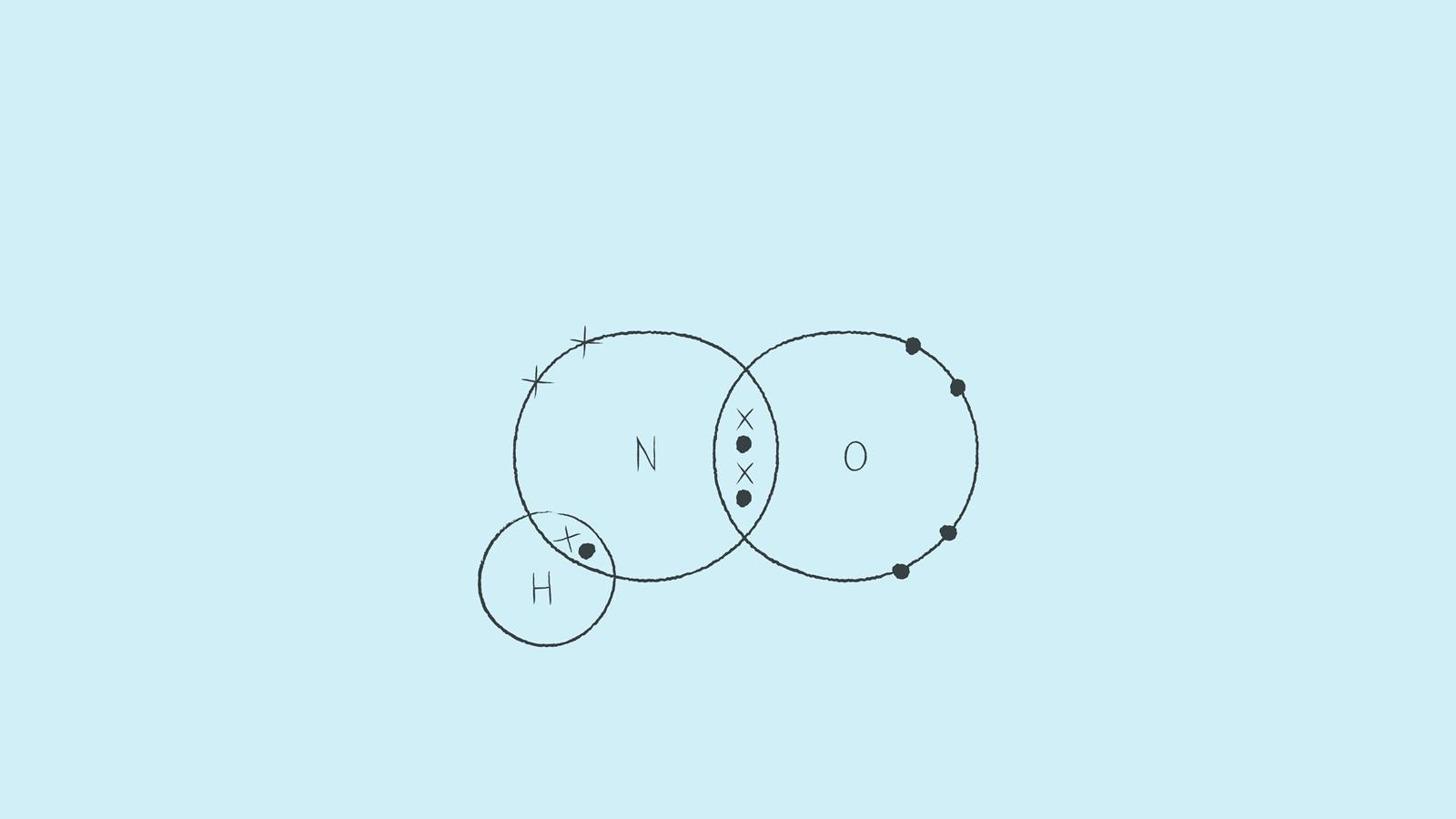
Nitroxyl contains a single covalent bond between hydrogen and nitrogen and a double covalent bond between nitrogen and oxygen.
Draw the dot and cross diagram with the outer shells overlapping. Then draw the shared electrons inside the overlapping section.
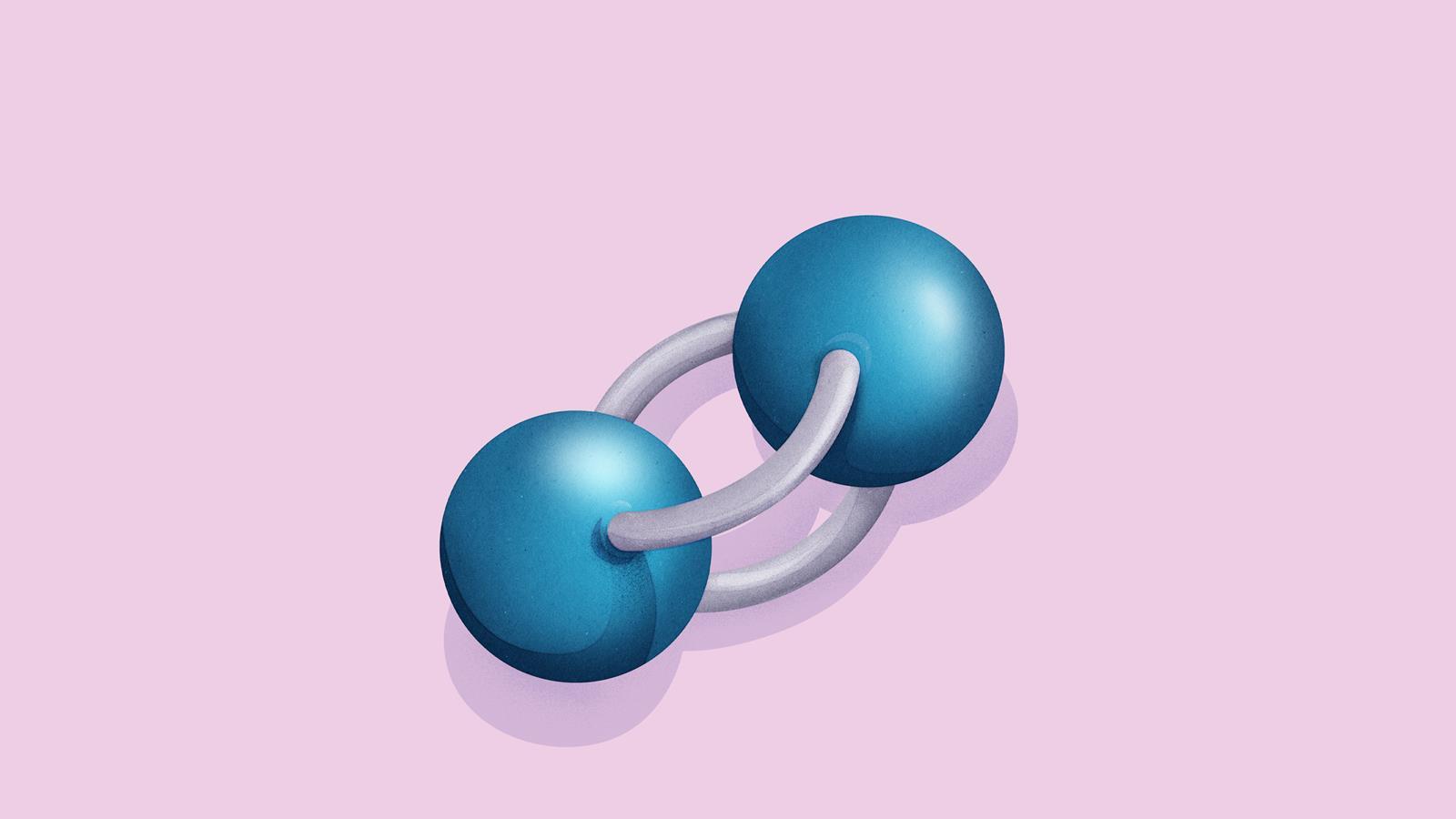
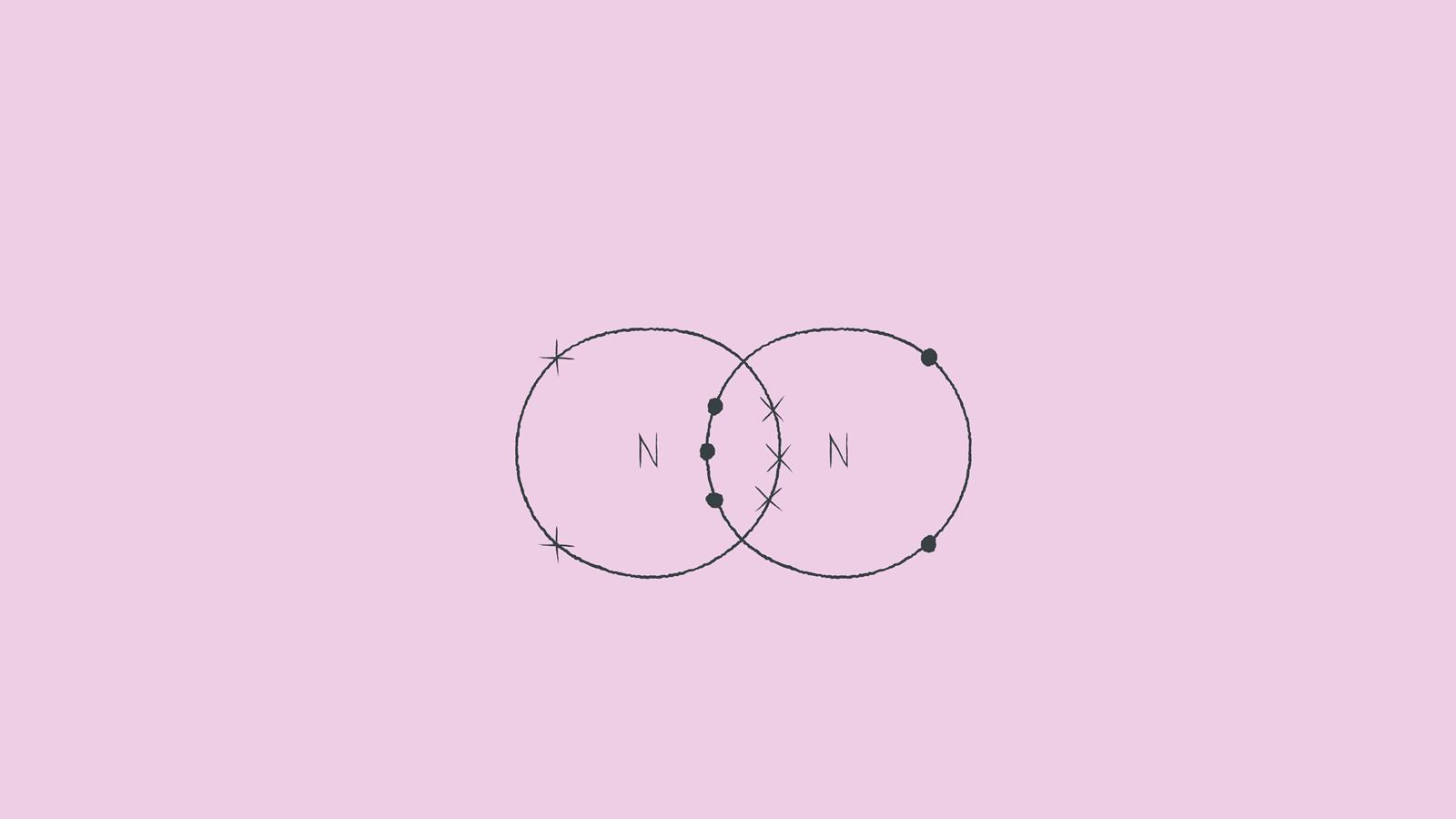
Nitrogen gas occurs naturally as a diatomic molecule. The bond between the two nitrogen atoms is a triple bond.
Again, draw the dot and cross diagram with the outer shells overlapping. However, this time draw the electrons on the lines rather than in the space between.
Want another step-by-step guide to drawing bonds? Check out this article with resources on drawing dot and cross diagrams for ionic bonding.
All illustrations © Dan Bright
Downloads
Dot and cross diagrams poster
PDF, Size 0.91 mbDot and cross diagrams fact sheet
Editable handout | Word, Size 0.42 mbDot and cross diagrams fact sheet
Handout | PDF, Size 0.12 mbCovalent bonding tiles student sheet
Editable handout | Word, Size 2.56 mbCovalent bonding tiles student sheet
Handout | PDF, Size 0.9 mbCovalent bonding tiles teacher notes
Editable handout | Word, Size 2.19 mbCovalent bonding tiles teacher notes
Handout | PDF, Size 3.43 mb





































1 Reader's comment