Learn about acid/base reactions as sodium hydroxide reacts with gas in the air to produce a solution that appears to disappear
Much like a spy, the chemists work is never done, and with this disappearing ink practical young learners can be both.
This experiment should take 30 minutes.
Equipment
Apparatus
- Eye protection
- Beaker, 100 cm3
- Measuring cylinder, 10 cm3
- Small paint brush to test the ink
Chemicals
- Ethanol
- Sodium hydroxide 0.4 mol dm–3
- Thymolphthalein solution (50 per cent water, 50 per cent ethanol)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Always wear eye protection.
- Ethanol is highly flammable, see CLEAPSS Hazcard HC040a.
- Sodium hydroxide is an irritant, see CLEAPSS Hazcard HC091a.
- Thymolphthalein soluiton is flammable, see CLEAPSS Hazcard HC032.
Procedure
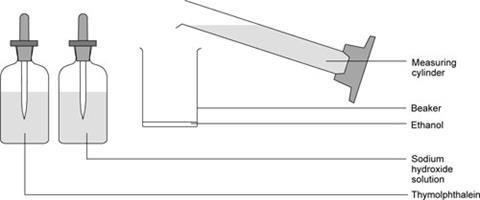
- Place 10 cm3 of ethanol in a small beaker.
- Add a few drops of thymolphthalein indicator solution.
- Add just enough NaOH solution, dropwise, to produce a deep blue colour in the solution.
- Using a small paint brush, test the ‘disappearing ink’ on a white page.
Notes
- This ink is the same as those sold in trick and joke shops.
- The amount of indicator can be adjusted to give a deep blue colour.
- The compound produced, Na2CO3, is commonly called washing soda.
- Sodium hydroxide reacts with carbon dioxide in the air to form sodium carbonate.
- 2NaOH(aq) + CO2(g) → Na2CO3(aq) +H2O(l)
- Sodium carbonate is less basic than sodium hydroxide and causes the indicator to change from blue to colourless.
- The colourless range for thymolphthalein is below pH 9.3.
- The blue range is above pH 10.5 and the colour change takes place between these two.
- The alcohol evaporates and leaves a clear and colourless residue.
Questions
The colour change occurs because sodium hydroxide reacts with a gas in the air.
- Which gas in the air causes this colour change?
- Write a word equation for the reaction.
- Write a formula equation for the reaction.
Answers
- Carbon dioxide
- Sodium hydroxide + carbon dioxide → sodium carbonate + water
- 2NaOH + CO2 → Na2CO3 + H2O
Downloads
Disappearing ink - teacher notes
PDF, Size 0.16 mbDisappearing ink - student sheet
PDF, Size 0.16 mb
Additional information
This practical is part of our Classic chemistry experiments collection.


















No comments yet