Introduce your students to the study of electrolysis by demonstrating how conduction is only possible where lead(II) bromide is molten, and that metallic lead and bromine are the products of the molten electrolyte
This demonstration does not need too much preparation and the apparatus involved is very straightforward. It must be done in a fume cupboard as bromine (VERY TOXIC and CORROSIVE) is produced. A safer alternative may be the Electrolysis of molten zinc chloride. The demonstration takes about 30–40 mins.
Equipment
Apparatus
- Eye protection (for students and demonstrating teacher)
- Access to a fume cupboard
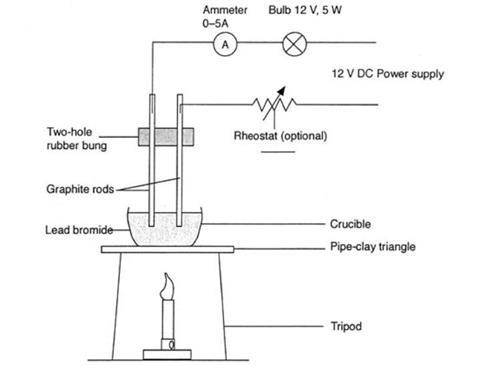
- Porcelain crucibles, about 2.5 cm in diameter, x2
- Pipeclay triangle
- Graphite rod electrodes, about 15 cm long, x2
- Rubber bung with two holes about 1 cm apart to fit the graphite rods
- DC power supply adjustable to 12 V (note 1)
- Ammeter, 0–5 A, ideally a large demonstration model
- Leads and crocodile clips
- Pair of metal tongs
- Large wooden board such as dissecting board
Apparatus notes
- You will need a rheostat if the power pack is not adjustable. A 12 V, 5 W bulb mounted on a holder could be used if a large ammeter is unavailable.
Chemicals
- Lead(II) bromide (TOXIC), about 20 g
- Universal indicator (FLAMMABLE), a few drops
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection and consider wearing gloves when handling the lead bromide. Wash hands after the demonstration.
- Lead(II) bromide, PbBr2 (s), (TOXIC, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC057a.
- Bromine, Br2 (l) and (g), (VERY TOXIC, CORROSIVE, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC015a.
Procedure
Before the demonstration
- Assemble the apparatus in a fume cupboard. During the experiment highly toxic bromine vapour is produced.
- The students should be given an opportunity to see the melting lead(II) bromide, and to observe that no conduction is possible until it melts.
- The graphite rods should reach almost to the bottom of the crucible, because the lead(II) bromide shrinks considerably when it is melted. This is why the crucible should be filled to overflowing with lead(II) bromide powder. Gloves should be worn when handling the lead(II) bromide. Ensure that the two-holed bung is as far up the graphite rods as possible, to prevent it burning or melting when strong heat is applied from below.
The demonstration
- Safety glasses must be worn throughout this demonstration.
- Set the power supply at 12 V, and short-circuit the rods to demonstrate that the circuit is working. Separate the rods and lower them into the powder. Demonstrate that the solid electrolyte does not conduct.
- Light the Bunsen burner and use a roaring flame to heat the crucible. The powder will take a few minutes to melt. This is because lead(II) bromide is not a good conductor of heat, and has a high thermal capacity. Eventually the powder melts and the current flows.
- Continue heating the crucible. The current rises as more and more of the solid melts. If necessary, top up the crucible with fresh powder. When all the powder has melted, use the power supply and/or rheostat to adjust the current to about 1.5 A.
- If a white sheet is placed behind the apparatus, the brown colour of the bromine will be seen. If a piece of moist universal indicator paper is briefly held in the vapour, it will turn red and may also be partially bleached. Small groups of students can file past the apparatus to confirm that the bromine is being evolved from the anode, and that no gas is evolved from the cathode. They too can do the test with universal indicator paper.
- Continue to maintain the current at about 1.5 A for 15 minutes. At the end of this time remove the heat from the apparatus, switch off the power supply and remove the graphite rods from the crucible. Use the tongs to pour the molten lead bromide into the second crucible, while keeping the small bead of molten lead in the first. Allow the crucibles to cool.
- When cool, remove the solidified metallic bead of lead and transfer it to a ceramic mat. This bead can be passed amongst the students, who can verify that it can be marked with a fingernail. Students who touch the metal must wash their hands afterwards.
Teaching notes
The electrolysis of molten salts is not as complicated as in aqueous solutions, because the electrolysis products of water are not an issue.
Although the graphite rods should be clamped as low as possible in the crucible, they should not be so low as to risk a short-circuit in the pool of liquid lead which collects at the bottom.
The solidified lead(II) bromide can be scraped out of the second crucible afterwards, so that it can be used again for this demonstration.
Lead(II) bromide is used because it melts at an unusually low temperature for an ionic compound (373 °C). Lead can be fairly safely handled afterwards (taking care to ensure that hands are washed after any contact with the metal). Bromine is a coloured acidic gas with a characteristic smell.
Electrolysis is not possible with solid lead(II) bromide. This is because the ions are held in a three-dimensional lattice, unable to move freely to the electrodes. Melting enables the ions to become mobile and to travel to the respective electrodes.
At the cathode (-) molten lead is formed:
Pb2+(l) + 2e-→ Pb(l)
At the anode, gaseous bromine is evolved:
2Br-(l) → Br2(g) + 2e-
If there is time, you may like to show that heating alone is insufficient to cause the lead(II) bromide to decompose.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet