Test for oxygen gas produced from the reaction between hydrogen peroxide and potassium manganate (VII) using methylene blue solution
In this experiment, students will create a gas generating apparatus with a set of pipettes and rubber tubing, to produce a reaction between hydrogen peroxide, and potassium manganate. They can then test for oxygen using methylene blue solution, noting down their findings.
This experiment should take 15 minutes.
Equipment
Apparatus
- Eye protection
- Student worksheet
- Beaker, 10 cm3
- Plastic pipette (standard form)
- Piece of rubber tubing, ca 10 cm long
- Scissors
Chemicals
Solutions should be contained in plastic pipettes. See the accompanying guidance on apparatus and techniques for microscale chemistry, which includes instructions for preparing a variety of solutions.
- Hydrogen peroxide 5% solution
- Potassium manganate(VII), 0.1 mol dm–3
- Methylene blue solution (colourless, leuco form of dye)
- Glucose
Health, safety and technical notes
- Wear eye protection throughout (splash-resistant goggles to BS EN166 3).
- Glucose / methylene blue / sodium hydroxide solution is corrosive, goggles (to BS EN166 3) should be worn. Reducing the concentration to below 0.5 mol dm-3 will mean it is merely an irritant and will still work (see CLEAPSS Hazcard HC040c, HC032).
- Sodium hydroxide itself is highly corrosive, if students are making up their own solutions (see CLEAPSS Hazcard HC091a).
- Hydrogen peroxide, 5% solution H2O2(aq) and potassium manganate(VII), KMnO4(aq), 0.1 mol dm–3 are of low hazard (see CLEAPSS Hazcard HC050, HC081).
- Using less (2.4 g) of potassium hydroxide will mean the solution is irritant rather than corrosive, and pupils can just use safety glasses (see CLEAPSS Hazcard HC091b).
Procedure
- Construct the gas generating apparatus by cutting the top off a plastic pipette so that a piece of rubber tubing can be attached to the pipette as shown:
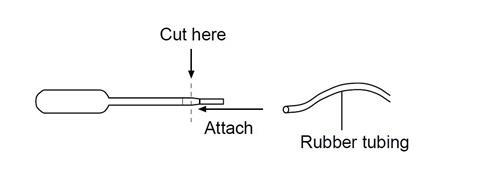
- Add methylene blue solution to a 10 cm3 beaker until it is about half-full.
- Add 10 drops of hydrogen peroxide to the shortened pipette.
- Turn the pipette almost to the horizontal position and carefully put five drops of potassium manganate(VII) solution in the stem as shown:
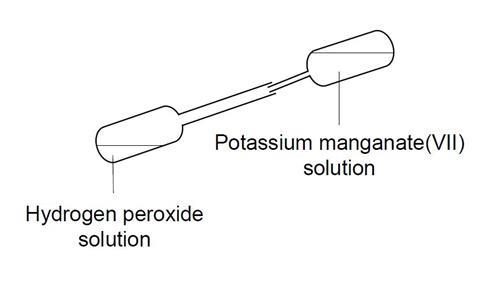
- Attach the rubber tubing to the pipette, place the other end in the methylene blue solution, and gently turn the pipette upright. The potassium manganate(VII) solution, held in the stem, should fall down into the hydrogen peroxide, causing vigorous evolution of oxygen gas.
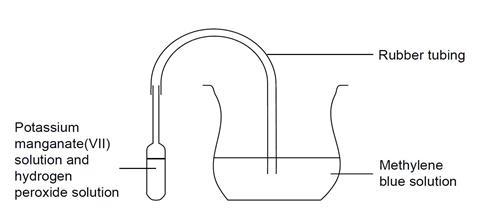
- Describe your observations.
Observations
This experiment is a little tricky to perform and students will need to practice it first! The hydrogen peroxide and potassium manganate(VII) react together vigorously to produce oxygen gas.
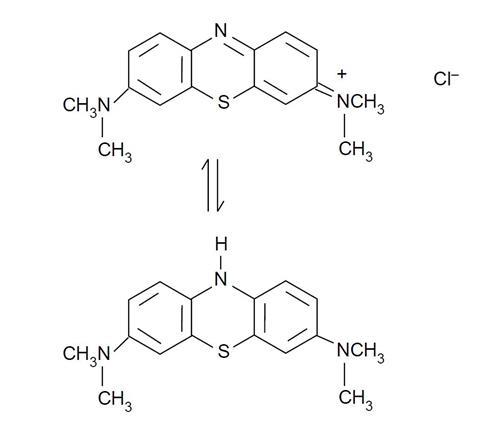
The colourless solution of methylene blue should turn blue quickly when the oxygen gas is directed into it. Students are given the structures of the oxidised and reduced forms of methylene blue and are asked to say which is which.
The oxidised (blue) form contains conjugated double and single bonds throughout the whole molecule, whereas in the colourless form the delocalised electron systems are isolated from each other. The structures are given below.
Student questions
The reaction (above) shows the structures of methylene blue in the reduced (colourless) and blue (oxidised) forms.
- Which structure is which?
- Can you give reasons for your answer?
- Can you write an equation for the reaction between potassium manganate(VII) and hydrogen peroxide?
Notes
Dissolve 4 g of potassium hydroxide pellets in 150 cm3 of deionised water in a plastic bottle or stoppered 250 cm3 conical flask. Allow to cool and add 5 g of glucose powder. Add 3–4 drops of methylene blue solution (0.25 g in 1000 cm3 of deionised water or Aldrich cat. no. 31,911-2). The blue solution should become colourless on standing a few minutes, but will turn blue when shaken.
Downloads
Oxygen and methylene blue – teacher notes
PDF, Size 0.2 mbOxygen and methylene blue – teacher notes
Word, Size 0.1 mbOxygen and methylene blue – student sheet
PDF, Size 0.29 mbOxygen and methylene blue – student sheet
Word, Size 0.22 mb
References
S. W. Breuer, Microscale practical organic chemistry. Lancaster: Lancaster University, 1991.
Additional information
This resource is part of our Microscale chemistry collection, which brings together smaller-scale experiments to engage your students and explore key chemical ideas. The resources originally appeared in the book Microscale chemistry: experiments in miniature, published by the Royal Society of Chemistry in 1998.
© Royal Society of Chemistry
Health and safety checked, 2018


















No comments yet