This microscale synthesis will highlight the structure of indigo dye, showing students how it is made, and giving them the chance to create their own
Indigo dye has been produced commercially for hundreds of years, but its history extends to pre-Roman Britain. Students will learn about its history, use and formation in this experiment which allows them to synthesis their own indigo dye.
This experiment should take 10 minutes.
Equipment
Apparatus
- Eye protection
- Test tube, or beaker, 10 cm3
- Small filter funnel
- Filter paper
Chemicals
Solutions should be contained in plastic pipettes. See the accompanying guidance on apparatus and techniques for microscale chemistry, which includes instructions for preparing a variety of solutions.
- Sodium hydroxide 0.5 mol dm–3
- Deionised water
- Propanone
- 2-Nitrobenzaldehyde
Health, safety, and technical notes
- Read our standard health and safety guidance.
- Students must wear suitable protection (Splash resistant goggles to BS EN166 3).
- Sodium hydroxide solution, NaOH(aq), 0.5 mol dm–3 is corrosive (see CLEAPSS Hazcard HC091a).
- Propanone is highly flammable, and an eye/respiratory irritant (see CLEAPSS Hazcard HC085a)
- 2-Nitrobenzaldehyde is Harmful if swallowed and a skin/eye and respiratory irritant (see ChemSpider10630).
Procedure
- Weigh out approximately 0.1 g of 2-nitrobenzaldehyde into a test tube.
- Add 2 cm3 of propanone (from a measuring cylinder) and swirl gently to dissolve the solid.
- Add 25 drops of deionised water and swirl gently.
- Slowly add 20 drops of sodium hydroxide solution to the solution. The solution quickly darkens and a purple solid (indigo) should precipitate out.
- Leave for 5 minutes to complete the precipitation.
- Filter the solution washing with deionised water until the washings are colourless, and set aside to dry.
- Describe the appearance of your product.
Observations
2-Nitrobenzaldehyde dissolves in propanone to form a pale yellow solution. When sodium hydroxide solution is added, the solution darkens after a few seconds and a purple precipitate of indigo forms.
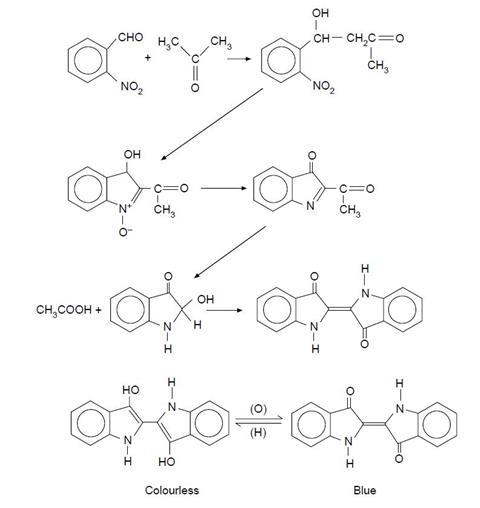
The synthesis is very simple and quick to perform, yet it is mechanistically complex, involving a series of condensations, disproportionations and oxidations. The sequence of reactions is given in the Journal of Chemical Education and this, together with the structures of the two forms of indigo.
There is one unusual step which produces ethanoic acid and what appears to be a hydroxy-derivative of indoxyl prior to the final oxidation to form indigo (indigotin). It is worthwhile pointing out to students that it took Baeyer 14 years (from 1865 to 1879) to find a route for the original synthesis of indigo and even when he did, it took him another four years to deduce the correct formula (in 1883).
The indigo can be filtered off and then dissolved in an alkaline solution of sodium dithionite to form the colourless, soluble form (leucoindigo). A piece of cotton cloth dipped into this solution and then exposed to air produces the indigo-dyed fabric – this is the procedure used in vat dyeing.
Downloads
The microscale synthesis of indigo dye - teacher notes
PDF, Size 0.23 mbThe microscale synthesis of indigo dye - teacher notes
Word, Size 0.11 mbThe microscale synthesis of indigo dye - student sheet
PDF, Size 0.16 mbThe microscale synthesis of indigo dye - student sheet
Word, Size 81.18 kb
References
S. W. Breuer, Microscale practical organic chemistry. Lancaster: Lancaster University, 1991.
Additional information
This resource is part of our Microscale chemistry collection, which brings together smaller-scale experiments to engage your students and explore key chemical ideas. The resources originally appeared in the book Microscale chemistry: experiments in miniature, published by the Royal Society of Chemistry in 1998.
© Royal Society of Chemistry
Health and safety checked, 2018


















No comments yet