All Redox chemistry articles – Page 6
-
 Class experiment
Class experimentElectrolysis of copper(II) sulfate solution
Explore the electrolysis of copper(II) sulfate solution and related industrial processes with this class experiment. Includes kit list and safety instructions.
-
 Resource
ResourceA transient red colour: the aqueous chemistry between iron (III) ions and sulfur oxoanions
An exploration into bonding in sulphur oxoanions, and redox reactions. Contains kit list and safety instructions.
-
 Exhibition chemistry
Exhibition chemistryMagic beakers
Declan Fleming shows you how to capture your students’ imaginations with spectacular demonstrations
-

-
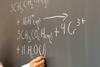
-
 Exhibition chemistry
Exhibition chemistryBeyond the ‘blue bottle’
Using indigo carmine to produce a range of stunning colours
-
 Exhibition chemistry
Exhibition chemistryBalls of fire!
Another spectacular demonstration of the dehydrating properties of acid
-
 The Mole
The MoleBatteries and electrochemical cells
Investigate the chemistry behind the battery in your smartphone and find out how you can build a simple electrochemical cell from everyday items in your house.
-
 Exhibition chemistry
Exhibition chemistryA visible reduction
Declan Fleming shows you how to capture your students’ imaginations with spectacular demonstrations
-
 Class experiment
Class experimentElectrochromic polymer
Can polymers conduct electricity as well as metals? Using this experiment, learners will discover how the two materials differ. Includes kit list and safety instructions
-
 Resource
ResourceWhich bleach is the best buy?
Which bleach is best? By investigating this question, learners will discover more about redox reactions. Includes kit list and safety instruction.
-
 The Mole
The MoleWhat is vitamin C and how can we test for it?
Find out about vitamin C or ascorbic acid and try a short experiment to test for its presence using iodine in this article from our ‘Avogadro’s lab’ series.
-
 Feature
FeaturePaper conservation
History is written on paper and chemistry is at the heart of paper conservation
-

-
![Feature microscale pg022 fig5 250 tcm18 216607[1]](https://d1ymz67w5raq8g.cloudfront.net/Pictures/100x67/9/4/2/113942_feature_microscale_pg022fig5_250_tcm182166071_353125.jpg) Feature
FeatureMicroscale chemistry revisited
Microscale techniques are unlikely to replace our traditional approach to chemistry education, but they do provide an extra dimension to our teaching strategies
-

-
 Exhibition chemistry
Exhibition chemistryDissolving copper in nitric acid
The dramatic reaction between copper and nitric acid ought to be seen
-

-

-
 Exhibition chemistry
Exhibition chemistryThe bizarre oscillating redox reaction between mercury and iron
Demonstrations designed to capture the student's imagination











