Investigate the effect of transition metal catalysts on the reaction between iron(III) nitrate and sodium thiosulfate
In this experiment, students compare the rate of reaction between iron(III) nitrate solution and sodium thiosulfate solution when different transition metal ions are used as catalysts. The catalysts used are copper(II), cobalt(II) and iron(II) ions.
Iron(III) ions are reduced to iron(II) ions in the presence of sodium thiosulfate. The reaction proceeds via a dark violet unstable complex but gives a colourless solution with time.
Students can do this experiment in pairs or small groups. If each pair of students attempts this experiment, large volumes of both the iron(III) nitrate solution and the sodium thiosulfate solution will be required.
Equipment
Apparatus
- Eye protection
- Stopclock or timer
- Dropping pipette (see note 9 below)
- Glass measuring cylinder, 100 cm3
- Measuring cylinder, 50 cm3
Chemicals
Access to 0.1 M solutions of the following (see note 8 below):
- Cobalt(II) chloride solution, (TOXIC), drops
- Copper(II) sulfate solution, drops
- Iron(II) sulfate solution, drops
- Iron(III) nitrate solution, 250 cm3
- Sodium thiosulfate solution, 250 cm3
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Cobalt(II) chloride solution, CoCl2 (aq), (TOXIC) – see CLEAPSS Hazcard HC025 and Recipe Book RB030.
- Copper(II) sulfate solution, CuSO4 (aq) – see CLEAPSS Hazcard HC027c and Recipe Book RB031.
- Iron(II) sulfate, FeSO4 (aq) – see CLEAPSS Hazcard HC055B and Recipe Book RB051.
- Iron(III) nitrate solution, Fe(NO3)3 (aq) – see CLEAPSS Hazcard HC055C and Recipe Book RB052. If iron(III) nitrate is not available, iron(III) chloride, 0.1 M , or iron(III) ammonium sulfate, 0.1 M, can be used instead.
- Sodium thiosulfate solution, Na2 S2 O3 (aq) – see CLEAPSS Hazcard HC095A and Recipe Book RB087.
- It is important that the concentrations of the solutions are accurate. If higher concentrations are used the experiment proceeds too quickly. It is useful if each group of students has access to their own supply of solutions, this prevents contaminating the bulk supply. The catalyst solutions can be provided in dropping bottles and the iron(III) nitrate and sodium thiosulfate solutions in 500 cm3 beakers.
- Use the type of teat pipette usually fitted to universal indicator bottles that does not allow squirting.
Procedure
- Draw a cross on a piece of scrap paper and put it underneath the 100 cm3 measuring cylinder so it can be seen when looking down the cylinder from the top.
- Using the 100 cm3 measuring cylinder, measure 50 cm3 of sodium thiosulfate solution. Place the cylinder back on top of the cross.
- Using a 50 cm3 measuring cylinder, measure 50 cm3 of iron(III) nitrate solution.
- Pour the iron(III) nitrate solution into the sodium thiosulfate solution, and start the timer. An immediate dark violet solution is observed which turns colourless after a few minutes.
- Look through the reaction mixture from above until the cross can first be seen. Stop the timer and record the time.
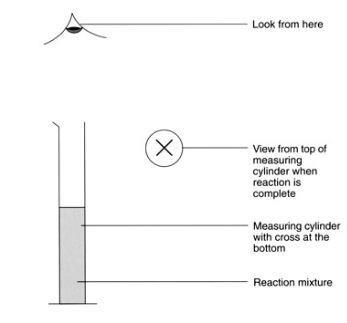
- Repeat this experiment, but add one drop of catalyst to the iron(III) nitrate solution before mixing. Test the various catalysts in the same way.
- Record the times for no catalyst and all the catalysts tested.
Teaching notes
Initially the iron(III) and thiosulfate ions form an unstable complex (which is dark violet in colour):
Fe3+(aq) + 2S2O32–(aq)→ [Fe(S2O3)2(H2O)2]–(aq)
Over time the complex is consumed as thiosulfate (acting as a reducing agent) reduces iron(III) to iron(II) ions. Transition metal ions can catalyse this reduction process at different rates.
If too much catalyst is used then the reaction proceeds instantaneously. It is important that students only use one drop of catalyst.
It is possible to set up this experiment using a light sensor and data logging. The data logging software should show the colour change occurring on a graph. This gives more information than the standard end point approach. The rate of change can be measured from the slope of the graph or the time taken for the reaction to occur.
Student questions
Some possible questions to ask students include:
- Which is the best catalyst?
- Why were only very dilute solutions of the catalysts used?
- Could you slow the reaction down? If so, how?
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet