Support learners to retrieve information on the diagrams used to represent ionic bonding
This resource is part of our support for literacy in science teaching, designed to embed literacy into your curriculum and develop learners’ skills in reading, writing and talking about science and their understanding of scientific language.
-

Download this
Download the structure strips, which are printed five to a page, to support learners to find or retrieve information and write independently.
View and download more Structure strips
Learning objectives
- Describe ion formation.
- Draw dot and cross diagrams of ionic bonding and explain how an ionic lattice is held together.
- State the limitations of a range of models used to represent ionic bonding.
Introduction
Ionic bonding occurs when atoms lose and gain electrons to form ions and then the positively and negatively charged ions are attracted to each other. In this activity, learners will gain an understanding of the models and diagrams used to represent this and their limitations.
How to use structure strips
Structure strips are a type of scaffolding you can use to support learners to retrieve information independently. Use them to take an overview at the start of the topic, to activate prior knowledge, or to summarise learning at the end of a teaching topic. For more ideas on how to use structure strips with your learners, see 5 ways to use structure strips effectively
Structure strips have sections containing prompts which are sized to suggest the amount that learners must write. Learners glue the strips into the margin of an exercise book and write their answers next to the sections, in full sentences. When learners have finished using the structure strip, they should have an A4 page set of notes and examples.
The strips are printed five to a page and will need to be trimmed to size. Find them on the second page of the student sheet.
Scaffolding
To further support learners to answer the questions you can include a list of keywords or add prompts to the structure strip.
As learners grow in confidence, they may be able to answer the question without the structure strip or attempt the question first and then use the structure strip to improve or self-assess their answer.
Metacognition
This activity supports learners to develop their metacognitive skills in the three key areas:
- Planning: the strips provide scaffolding to plan the written response. Learners will decide where to gather information from (eg textbooks, own notes, revision websites). Ask learners: is the source of information I am using reliable?
- Monitoring: learners are prompted by the questions in the structure strip and can check their answer against the prompts. Ask learners: have you covered all of the questions in the space provided? Do you need to change anything to complete the task?
- Evaluation: learners can self-assess or ask a peer to check their work against the answers. Ask learners: did you achieve what you meant to achieve? What might you do differently next time?
Keywords
Bonding, dot and cross diagram. electron, ionic, ions, lattice, limitation, negative, positive.
Follow-up question
Learners answer this question after attempting the structure strip. The structure strip activates the required knowledge which learners can then apply to this question.
Draw a dot and cross diagram to show what happens when atoms of sodium and oxygen react to produce sodium oxide. Discuss the advantages and limitations of the dot and cross diagram for this compound.
Answers
Suggested answers for the structure strip activity are given in the downloadable teacher notes.
Answers to follow-up question:
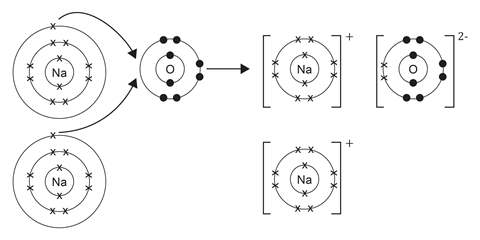
Explanation of charges: Each sodium ion has lost one electron, it now has 11 protons (+ charge) and 10 electrons (- charge) so has an excess charge of +1 leading to a 1+ charge on the ions.
The oxide ion has gained two electrons (one from each of the two sodium atoms), it now has eight protons (+ charge) and 10 electrons (- charge) so has an excess charge of -2 leading to a 2- charge on the ion.
Evaluation of models: Dot and cross diagrams are good as they show what has happened in terms of electron transfer and make it clear that electrons have been lost and gained. They are limited because they do not show the giant lattice structure.
Downloads
Ionic bonding structure strips student sheet
Handout | PDF, Size 0.18 mbIonic bonding structure strips teacher notes and answers
Handout | PDF, Size 0.36 mbIonic bonding structures strips student sheet
Editable handout | Word, Size 0.46 mbIonic bonding structure strips teacher notes and answers
Editable handout | Word, Size 0.59 mb

































No comments yet