Try this class practical to make a pH indicator from red cabbage with your students
A pH indicator is a substance which has one colour when added to an acidic solution and a different colour when added to an alkaline solution. Various colouring materials in plants can act as indicators. In this practical, students make an indicator from red cabbage.
The experiment is in two parts. In the first part, students boil some red cabbage in water. In the second part, they test their indicator. Between the two parts the mixture must be allowed to cool. The first part takes about 10–15 minutes. The cooling takes about 15 minutes and the testing less than five minutes.
The cooling period could be used as an opportunity to discuss the background to the experiment – see Teaching notes below.
Equipment
Apparatus
- Eye protection
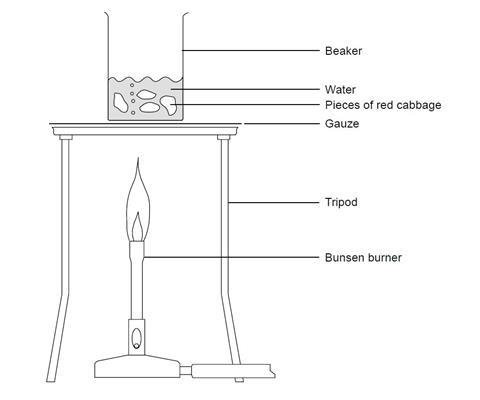
- Beaker, 250 cm3
- Bunsen burner
- Tripod
- Gauze
- Heat resistant mat
- Test tubes, x3 (small test tubes of about 10 cm3 are ideal)
- Test tube rack
- Dropper pipette
- Several pieces of red cabbage
Chemicals
Students will need access to:
- Dilute hydrochloric acid, 0.01 M
- Sodium hydroxide solution, 0.01 M
- Deionised or distilled water
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout. Consider clamping the beaker.
- Each group of students will need access to the hydrochloric acid and sodium hydroxide solutions, and to deionised or distilled water. Provide all three in similar containers labelled ‘Acid’, ‘Alkali’ and ‘Water’. Dropper bottles are ideal. Alternatively small beakers (100 cm3) with dropper pipettes could be used. Students need to be able to pour the acid and alkali solutions easily and safely into test tubes.
- Dilute hydrochloric acid, HCl(aq) – see CLEAPSS Hazcard HC047a.
- Sodium hydroxide solution, NaOH(aq) – see CLEAPSS Hazcard HC091a.
Procedure
- Boil about 50 cm3 of water in a beaker.
- Add 3 or 4 small (5 cm) pieces of red cabbage to the boiling water.
- Continue to boil the red cabbage in the water for about 5 minutes. The water should turn blue or green.
- Turn off the Bunsen burner and allow the beaker to cool for a few minutes.
- Place 3 test tubes in a test tube rack. Half-fill one of the test tubes with acid, one with alkali, and one with distilled or deionised water. Label the test tubes.
- Use a dropper pipette to add a few drops of the cabbage solution to each test tube. Note the colour of the cabbage solution in each of the three test tubes.
Teaching notes
Discussion points could include any or all of the following:
- Many plant colouring materials in berries, leaves and petals act as indicators.
- Some of these will not dissolve in water easily. A solvent other than water (eg ethanol) could be used, but it may be flammable. Discuss how the risk of fire can be reduced by using a beaker of hot water to heat the mixture.
- Possible variations on this experiment might include using beetroot, blackberries, raspberries, copper beech leaves, or onion skins in place of the red cabbage.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet