Purify alum as an example of obtaining a pure chemical from an impure sample in this class practical
It is often necessary to obtain pure solid chemicals from impure substances. In this experiment, students use filtration and evaporation to purify alum, with the opportunity to grow large crystals of the pure chemical.
If students have already carried out this type of experiment – eg obtaining salt from a mixture of sand and salt – it can be presented as a simple type of investigation.
This experiment can be carried out by groups of two or three and will take about one hour to complete.
Equipment
Apparatus
- Eye protection
- Beaker, 100 cm3
- Glass stirring rod
- Filter funnel
- Filter papers, x2
- Evaporating dish
- Clamp and stand
- Bunsen burner
- Heat resistant mat
- Tripod
- Gauze
- Forceps (optional)
- Watch glass (optional)
Chemicals
- Impure alum (hydrated aluminium potassium sulfate) about 5 g per group of students (see note 4 below)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout. Take care with hot apparatus and solutions.
- Alum, KAl(SO4)2.12H2 O – see CLEAPSS Hazcard HC002B.
- To prepare the impure solid, add a small amount of dry soil to a sample of alum. Ensure the soil is taken from an area unlikely to be contaminated (away from dogs or cats). Depending on the type of soil, particles may pass through the filter paper.
Procedure
- Use about 3–4 g of the impure solid – enough to cover the bottom of the beaker.
- Add water until the beaker is about one-quarter full of liquid (25 cm3).
- Place the beaker on a tripod and gauze, over a Bunsen burner, and heat the mixture stirring it frequently with a glass rod.
- When the mixture boils, stop heating and allow the mixture to cool a little.
- Filter the hot solution into the evaporating dish (see diagram below).
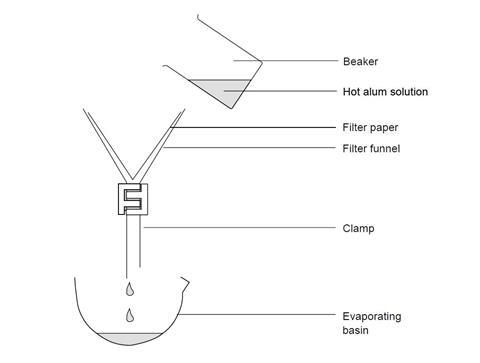
- Leave the solution to cool. If necessary cover the dish with a filter paper or watch glass and leave overnight.
- Remove the crystals by decanting or by using a pair of a forceps.
- Dry these crystals by dabbing them on pieces of dry filter paper.
Teaching notes
It is important that students use the quantities given above. Using too much solid will prevent all the alum from fully dissolving and a powdered, rather than a crystalline product will form. Using too much water means that on cooling, no crystals will appear, since the solution is still unsaturated.
Students should understand that this method works because alum dissolves in water whilst most of the foreign matter is insoluble. They should also realise that the alum eventually crystallises out since it is far less soluble in cold water than in hot.
This experiment can be extended by allowing students to ‘grow’ crystals from the saturated solution left behind in the evaporating dish. Traditionally this is carried out by suspending crystals from a length of cotton, but equally good results are obtained by choosing a well-formed crystal and placing it in some of the filtered saturated solution. Dust-free conditions are essential.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















1 Reader's comment