Colour in organic compounds is associated with unsaturation.
A carbon-carbon double bond in ethene, for example, absorbs energy as electrons in π bonds are promoted to higher energy levels. Bonds formed between carbon atoms in ethene are the result of overlap between atomic orbitals, producing bonding and antibonding molecular orbitals.
Orbitals are regions of space in which there is a 90% chance of finding electrons. However, in antibonding orbitals, which are of higher energy, electrons will not be found.
Transitions between orbitals of lower energy and antibonding orbitals occur when electromagnetic radiation of suitable energy is absorbed by the molecule.
In ethene, absorption of radiation of wavelength 171 nm is sufficient to promote electrons to π* anti-bonding orbitals.
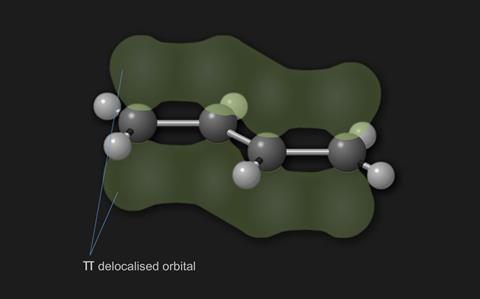
In buta-1,3-diene however, radiation of a longer wavelength (222 nm) will promote electrons. The reason for this difference is due to delocalisation of the electrons in the π bonds of buta-1,3-diene. When electrons occupy delocalised π bonds they are spread out over a larger volume and the energy associated with them is lowered. As the extent of delocalisation of electrons increases further, their energy lowers even more. Electrons can consequently be promoted more easily in extensively delocalised molecules and the wavelength of radiation concerned with the transition increases significantly.
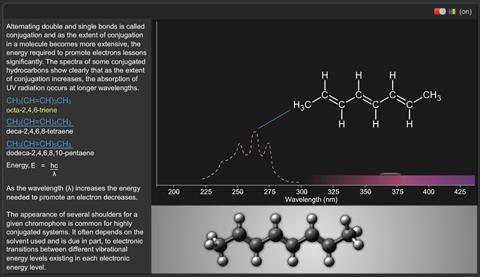
Alternating double and single bonds is called conjugation and as the extent of conjugation in a molecule becomes more extensive, the energy required to promote electrons lessens significantly. The spectra of some conjugated hydrocarbons show clearly that, as the extent of conjugation increases, the absorption of UV radiation occurs at longer wavelengths.
Octa-2,4,6-triene:
CH3 (CH=CH)3 CH3
Deca-2,4,6,8-tetraene
CH3 (CH=CH)4 CH3
Dodeca-2,4,6,8,10-pentaene
CH3 (CH=CH)5 CH3
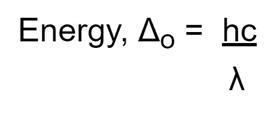
As the wavelength (λ) increases the energy needed to promote an electron decreases.
The appearance of several shoulders for a given chromophore is common for highly conjugated systems. It often depends on the solvent used and is due in part to electronic transitions between different vibrational energy levels existing in each electronic energy level.
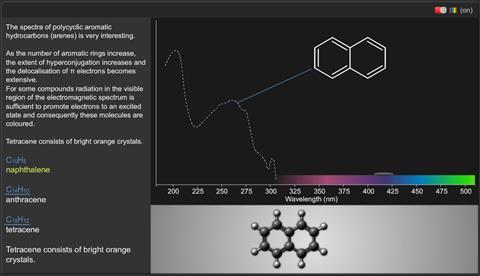
The spectra of polycyclic aromatic hydrocarbons (arenes) is very interesting. As the number of aromatic rings increase, the extent of hyperconjugation increases and the delocalisation of π electrons becomes extensive.
For some compounds radiation in the visible region of the electromagnetic spectrum is sufficient to promote electrons to an excited state and consequently these molecules are coloured.
Naphthalene:
C10 H8
Anthracene
C14 H10
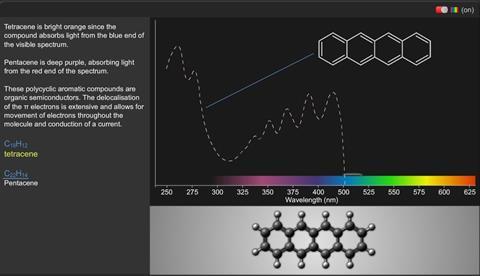
Tetracene:
C18 H12
Tetracene consists of bright orange crystals because the compound absorbs light from the blue end of the visible spectrum.
Pentacene
C22 H14
Pentacene is deep purple, absorbing light from the red end of the spectrum.
These polycyclic aromatic compounds are organic semiconductors. The delocalisation of the π electrons is extensive and allows for movement of electrons throughout the molecule and conduction of a current.
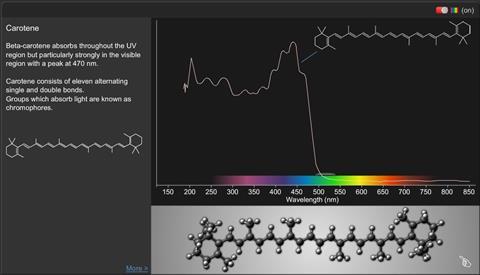
Carotene:
C40 H56
Beta-carotene absorbs throughout the UV region but particularly strongly in the visible region with a peak at 470 nm. Carotene consists of 11 alternating single and double bonds.
Groups which absorb light are known as chromophores.
Downloads
Bonding orbitals
Simulation | Flash, Size 9.62 kbBond energy transitions
Simulation | Flash, Size 4.87 kbDelocalisation
Simulation | Flash, Size 5.47 kbEthene bonding
Simulation | Flash, Size 5.05 kbButa-1,3-diene bonding
Simulation | Flash, Size 43.41 kbOcta-2,4,6-triene spectrum
Simulation | Flash, Size 0.32 mbNapthalene spectrum
Simulation | Flash, Size 0.28 mbTetracene spectrum
Simulation | Flash, Size 0.24 mbCarotene spectrum
Simulation | Flash, Size 0.35 mb
Ultraviolet–visible (UV-vis) spectroscopy
- 1
- 2
 Currently reading
Currently readingThe origin of colour in organic compounds
- 3
- 4




















1 Reader's comment