Consider a solution of chromium(III) nitrate;
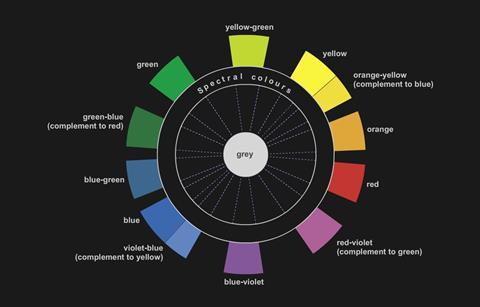
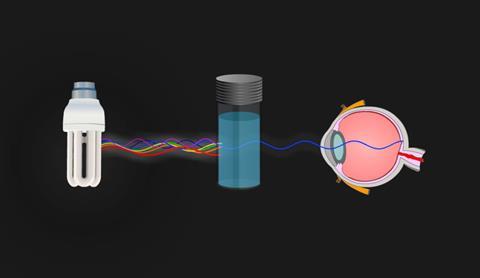
This solution is violet in colour because the solution absorbs yellow light. Yellow is the complimentary colour of violet.
Copper(II) sulfate solution appears blue because it absorbs orange-yellow light.
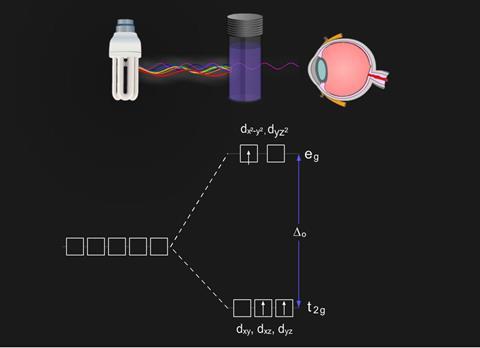
Cr3+ has three electrons, each singly occupying the t2g orbitals. When light is absorbed by the solution, an electron is promoted from a t2g orbital to a vacant, higher energy eg orbital.
Transition metal compounds are coloured because the energy involved with these transitions occurs in the visible part of the spectrum.
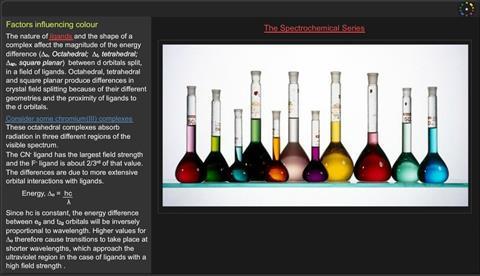
The nature of ligands and the shape of a complex affect the magnitude of the energy difference (Δo, Octahedral; Δt, tetrahedral; Δsp, square planar) between d orbitals split, in a field of ligands. Octahedral, tetrahedral and square planar produce differences in crystal field splitting because of their different geometries and the proximity of ligands to the d orbitals.
Consider some chromium(III) complexes:
- These octahedral complexes absorb radiation in three different regions of the visible spectrum.
- The CN ligand has the largest field strength and the F ligand is about 2/3rds of that value.
- The differences are due to more extensive orbital interactions with ligands.
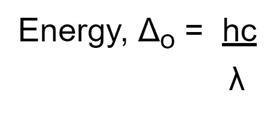
Since hc is constant, the energy difference between eg and t2g orbitals will be inversely proportional to wavelength. Higher values for Δo therefore cause transitions to take place at shorter wavelengths, which approach the ultraviolet region (in the case of ligands) with a high field strength .
The charge on a metal ion has a significant effect on crystal field splitting.
For the same ligand, a metal ion with a higher oxidation number will produce a greater crystal field splitting.
This is because the ligand will be more strongly attracted to the metal ion (which has a higher charge density) and will approach the d orbitals more closely, increasing the extent of repulsion and raising the magnitude of the energy difference (Δo) between the orbitals.
Solutions of iron(III) complexes are yellow or red whilst iron(II) complexes are pale green. This is because as the oxidation state of the metal increases, Δo increases and hence the frequency of the absorption moves towards higher frequency, i.e. for iron (III) the absorption moves towards the blue end (higher energy end) of the spectrum.
The Co(III) hexaammine complex has a Δo value which is about twice that of the Co(II) hexaammine complex.
For a given ligand and oxidation state, the magnitude of crystal field splitting increases down a group. Ions of metals in the first row of the transition series have lower Δo values than ions in the same group below. This is because, as the metal ions get larger the ligands are physically more remote from those orbitals with which they do not directly interact (i.e. t2g) and repel less. Hence the difference in energy between t2g and eg increases.
Downloads
Chromium(III) colour
Simulation | Flash, Size 9.44 kbComplementary colour wheel
Simulation | Flash, Size 9.32 kbCopper(II) sulfate colour
Simulation | Flash, Size 9.46 kbElectron promotion in Cr(III)
Simulation | Flash, Size 12.32 kbFactors influencing colour
Simulation | Flash, Size 0.63 mbFactors influencing colour (cont)
Simulation | Flash, Size 0.75 mb
Ultraviolet–visible (UV-vis) spectroscopy
Learn how UV-visible radiation can be used to shed light on chemical identification and how our senses percept colour. From the theory behind molecular orbitals and electronic transitions to the application of this technique with relatable examples. Includes examples and interactive simulations to aid understanding.
- 1
- 2
- 3
 Currently
reading
Currently
reading
Explanation of colour


























No comments yet