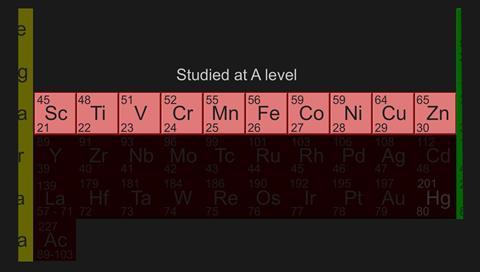
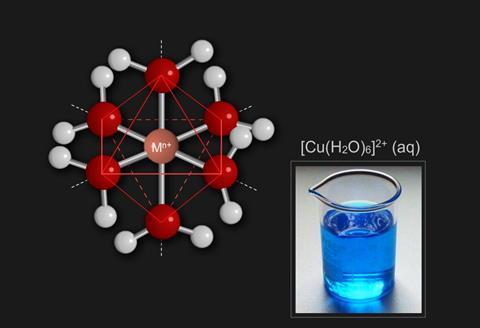
Transition elements are found in the d block of the periodic table and the most interesting feature of transition metal compounds is that most are highly coloured.
d block elements use s, p and d orbitals in bonding, forming complexes which exhibit a variety of oxidation states and involve other species called ligands.
To explain the reasons behind colour in transition metal complexes we need to briefly examine the nature of d orbitals and the way in which they interact with ligands.
- The bonds formed between transition metal ions and ligands are co-ordinate bonds.
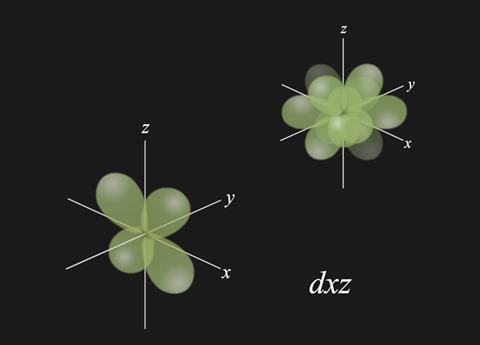
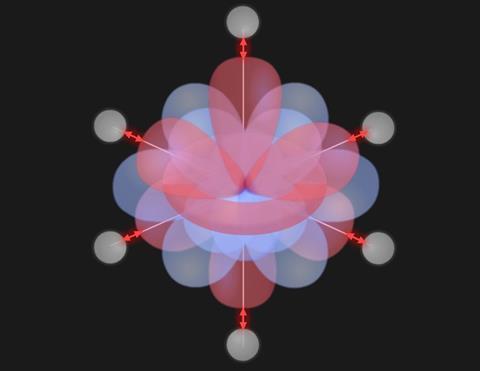
- There are five d orbitals which can each accommodate a pair of electrons:
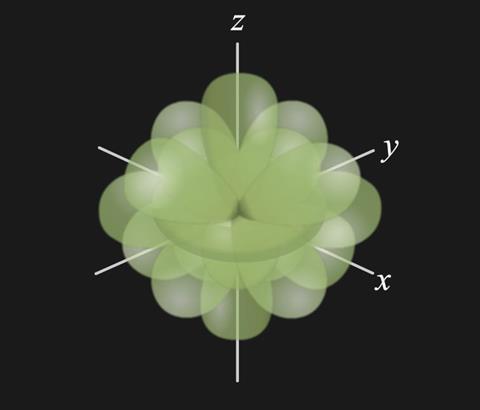
- The d orbitals can be considered to form a ‘sphere’ of charge around a transition metal ion:
Crystal Field Splitting
The majority of transition metal complexes are octahedral complexes, containing six ligands surrounding the central ion.Tetrahedral or square planar complexes are less common.
Ligands are attracted to the ion (having a positive charge) but they will be repelled by the d orbitals which contain electrons.
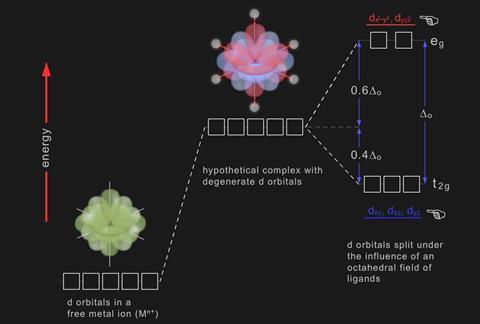
The ligand field which forms around the d orbitals causes the energy of the electrons in them to increase, but this increase is not the same for all of the d orbitals. For an octahedral complex, the energy of the orbitals is split into two. Three of the orbitals (t2g) are of lower energy and two have higher energy (eg).
The energy difference (Δo) is caused by the juxtaposition of the ligands and d orbitals.
The dz2 and dx2 – y2 orbitals line up with the ligands, creating greater repulsion and occupy higher energies whereas the remaining dxy, dyz and dxz reside in between the ligands.
Coloured complexes all contain from 1 – 9 d electrons. Complexes that are colourless do not contain metals with this particular electron configuration.
Downloads
Transition elements d-block
Simulation | Flash, Size 13.58 kbFive d orbitals
Simulation | Flash, Size 6.34 kbOrbitals as a sphere of charge
Simulation | Flash, Size 4.01 kbCFT ligands
Simulation | Flash, Size 0.12 mbCFT attracted to the ion
Simulation | Flash, Size 9.8 kbCrystal field splitting
Simulation | Flash, Size 20.69 kb
Ultraviolet–visible (UV-vis) spectroscopy
- 1
- 2
- 3
 Currently reading
Currently readingColour in transition metal compounds
- 4




















No comments yet