All Thermodynamics articles – Page 3
-
 Resource
ResourceRates and equilibria
This activity demonstrates the links between the topics of rates of reaction and the equilibrium law. It provides students with an explanation of the equilibrium law and helps them explain why Le Chatelier’s principle works for temperature, concentration and pressure.
-
 Resource
ResourceTheory v practice - do they compare?
Investigate the reactions of calcium metal, in theory and in practice. Does it act as you’d expect? Includes kit list and safety instructions.
-
 Feature
FeatureClearing the air around smoke formation
Answering the riddle of why unsaturated fuels burn with sooty flames
-
 Resource
ResourceThe Mpemba effect
Help discover why warm water freezes quicker than cold water. The Royal Society of Chemistry offered £1000 for the best and most creative explanation of this phenomenon, known as the Mpemba effect. View the winning entry, try the experiment for yourself, or read the original paper.
-
 Resource
ResourceInsulation and conduction: That’s Chemistry!
The ‘Insulation and conduction’ chapter from That’s Chemistry!: This chapter looks at key ideas and activities that can be used to help students learn how materials have different properties, including whether they are conductors or insulators of heat and/or electricity.
-

-

-

-
 Resource
ResourceThermodynamic quantities in a reaction
Calculate the thermodynamic quantities for a reaction using the standard enthalpy of neutralisation (strong acid and strong base) and any other information.
-

-
 Resource
ResourceThermodynamics and equilibria of the Solvay process
A worksheet with questions on the thermodynamics and acid-base aspects of the Solvay process and the uses of the products. This resource is part of the ‘Sodium carbonate – a versatile material’ resources collection, which presents learning material based on the manufacture and uses of sodium carbonate made by the ...
-
 Demonstration
DemonstrationThe sublimation of air freshener | 11–14 years
Use this experiment to demonstrate sublimation, showing how solid air freshener changes directly from a solid to a gas. Includes kit list and safety instructions.
-
 Resource
ResourceMaking biodiesel │16–18 years
Practical work on making a biodiesel, as well as worksheets covering alkenes, infrared spectroscopy, mass spectrometry, calculations on biodiesel yields, ester/biodiesel production and thermochemistry
-
 Demonstration
DemonstrationEndothermic solid–solid reactions
Observe an endothermic reaction between two solids in this demonstration or class experiment. Includes kit list and safety instructions.
-
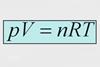 Resource
ResourceChemistry Vignettes: Physical Chemistry
A series of short screencasts to introduce aspects of physical chemistry.
-
 Resource
ResourceChemistry Vignettes: Advanced Physical Chemistry
A series of short screencast videos to introduce physical chemistry concepts.
-
 Feature
FeatureExploding some myths
Declan Fleming investigates what’s really going on when alkali metals hit water
-
 Demonstration
DemonstrationThe effect of pressure and temperature on equilibrium | Le Chatelier’s principle
Try this demonstration to explore the effects of pressure and temperature on an equilibrium mixture with your students. Includes kit list and safety instructions.
-
 Demonstration
DemonstrationThe effect of concentration and temperature on an equilibrium | Le Chatelier’s principle
Try this demonstration to illustrate how changing chlorine concentration or temperature shifts the position of an equilibrium. Includes kit list and safety instructions.
-
![I stock 000036213086 300tb[1]](https://d1ymz67w5raq8g.cloudfront.net/Pictures/100x67/5/7/8/113578_istock_000036213086_300tb1.jpg) Opinion
OpinionFive ideas in chemical education that must die: Le Châtelier’s principle
Eric Scerri examines Le Châtelier’s principle and shows how it can lead to confusion among students











