Quiz your students on the atomic structure of atoms
The topics covered in this Starter for ten activity are: development of theories about atomic structure; isoelectronic species; electrons and orbitals; and trends in ionisation energies.
Example questions
Our current understanding of atomic structure is a result of the discoveries of several scientists over many years, each scientist adding to the model.
Complete the table below by adding the name of the scientist and the discovery made.
Choose from the lists below the table.
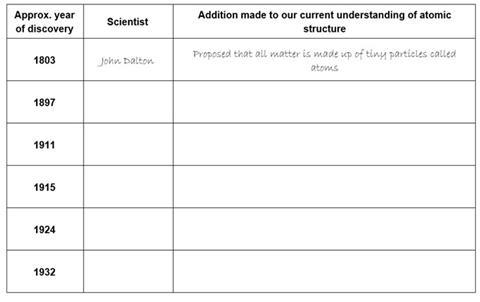
Discoveries;
Proposed that the electrons orbit around the nucleus in orbits with a set size and energy.
Discovered that atoms contain neutral particles called neutrons in their nucleus.
Realised that atoms are divisible and contain very tiny, negatively charged particles called electrons.
Discovered that an atom is made up of a nucleus and an extra-nuclear part. The central nucleus is positively charged and the negative electrons revolve around this central nucleus.
Proposed the concept of electron spin.
BONUS MARK: Which of the scientists listed above was a famous football goalkeeper in his country?
Notes
A full version of this resource, including questions and answers, is available from the ‘Downloads’ section below. An editable version is also available.
Downloads
Atomic structure – editable
Word, Size 0.36 mbAtomic structure
PDF, Size 0.38 mb
Starters for 10: Advanced level 1 (16–18)

This chapter in our Starters for ten series covers quantitative chemistry, atomic structure, bonding, trends in the periodic table, organic chemistry, thermodynamics, kinetics, equilibria, redox, analysis and experimental skills.
- 1
- 2
 Currently
reading
Currently
reading
Atomic structure
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12








































2 readers' comments