Identify aldehydes and ketones using Brady’s reagent (2,4-dinitrophenylhydrazine) in this microscale experiment
In this practical, students add various liquid aldehydes and ketones to 2,4-dinitrophenylhydrazine solution in a well-plate to form solid derivatives. They then do the same test with methanol and ethanol, showing that the reaction does not occur with alcohols.
See it in action!
You can see this test in our video Qualitative tests for organic functional groups (see 09:49), where it is used as part of a series of tests to identify six unknown organic compounds.
The experiment should take around 15 minutes.
Equipment
Apparatus
- Well-plate or spotting tile
For preparation of 2,4-dinitrophenylhydrazine
- Beaker, 100 cm3
Chemicals
Note
Solutions should be contained in plastic pipettes. See the accompanying guidance on apparatus and techniques for microscale chemistry, which includes instructions for preparing a variety of solutions.
- Ethanol
- Propanone
- p-Methoxybenzaldehyde (or other aromatic aldehyde or ketone)
- Methanol
- Ethanal (Acetaldehyde)
- Solution of 2,4-dinitrophenylhydrazine (see preparation notes below) – requires 24 hours to dissolve completely
For preparation of 2,4-dinitrophenylhydrazine
- Deionised water
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout (splash-resistant goggles to BS EN166 3).
- 2,4-dinitrophenylhydrazine, (NO2)2C6H3NHNH2(s) – see CLEAPSS Hazcard HC030 and CLEAPSS Recipe Book RB033. 2,4-dinitrophenylhydrazine is FLAMMABLE and HARMFUL if swallowed. It is also dangerous in contact with oxidising agents, and can potentially become explosive if allowed to dry out completely. Make only the minimum quantity as and when required and then promptly dispose of any unused reagent by diluting the content in a bucket of water and pouring down a foul-water drain with more water. Do not store the solution. Brady’s reagent can be purchased ready made from chemical suppliers. If using an existing stock of solid 2,4-DNPH, check that it has been stored in an outer container of water. Do not open a container that may contain dried out 2,4-DNPH. Avoid skin contact.
- Concentrated sulfuric acid, H2SO4(aq) – see CLEAPSS Hazcard HC098a and CLEAPSS RB098. Concentrated sulfuric acid is CORROSIVE and OXIDISING.
- Propanone – see CLEAPSS Hazcard HC085A and CLEAPSS Recipe Book RB002. Propanone is highly FLAMMABLE and IRRITANT to eyes and the respiratory system.
- p-Methoxybenzaldehyde is of low hazard.
- Methanol – see CLEAPSS Hazcard HC040b. Methanol is highly FLAMMABLE, TOXIC by all routes and causes damage to organs (particularly CNS and optic nerve). Propanol could be used as a safer alternative to methanol. It is FLAMMABLE (see CLEAPPS Hazcard HC084A).
- Ethanal (acetaldehyde) – see CLEAPSS Hazcard HC034. Ethanal is highly FLAMMABLE, a carcinogen and an eye/respiratory IRRITANT. Protect face when opening containers and ensure laboratory is well-ventilated. Avoid contact with sulfuric acid – violent polymerisation occurs.
- Well-plate or spotting tile – note that the precipitate formed may stain plastic well plates and spotting tiles.
To prepare 25 cm3 of solution of dinitrophenylhydrazine (2,4-DNPH)
Note that methods using concentrated sulfuric acid and methanol are no longer recommended. Wear splashproof goggles and chemical resistant gloves. Protect the bench to avoid staining.
- Add 0.5 g of 2,4-DNPH to about 12–13 cm3 of 85% phosphoric(v) acid. Do not use old phosphoric acid for the preparation of Brady’s reagent. Phosphoric acid undergoes polymerisation during long-term storage leading to the build-up of solid at the bottom of the bottle.
- Stir until the 2,4-DNPH has completely dissolved in the acid before moving on to Step 3. Dissolving may take 10–15 minutes.
- Once the 2,4-DNPH has dissolved, make up to a total volume of 25 cm3 with ethanol (or IDA). Stir to mix.
Procedure
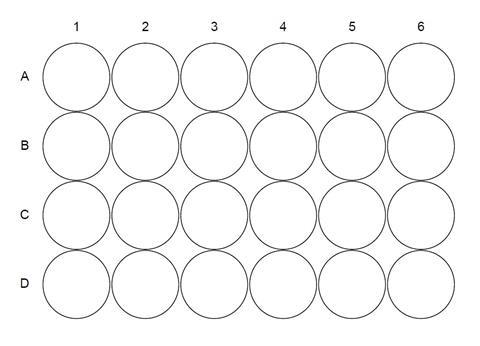
- In a spotting tile or well plate, add 10 drops of 2,4-dinitrophenylhydrazine solution to each of the wells A1–A5 (see diagram below).
- Carefully add three drops of ethanal to well A1 (ethanal is very volatile!).
- Repeat adding three drops of the other liquids to wells A2–A5.
- Observe any changes over the next few minutes.
Observations
| Compound | Observation |
|---|---|
| Ethanal | Immediate yellow precipitate |
| Propanone | Yellow, crystalline precipitate forms slowly (after 1–2 minutes) |
| p -Methoxybenzaldehyde (or other aromatic aldehyde or ketone) | Immediate red precipitate |
| Methanol | No change |
| Ethanol | No change |
Questions
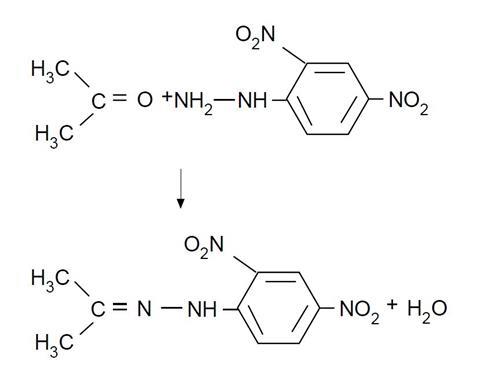
- Can you write equations for any reactions occurring?
- Why do you think that these reactions serve such a useful purpose in identifying aldehydes and ketones?
- Aldehydes and ketones will also form derivatives with hydrazine itself. What is the purpose of using 2,4-dinitrophenylhydrazine in this experiment instead of hydrazine?
More resources
Inspire learners and discover more ways chemists are making a difference to our world with our video job profiles.
Downloads
Brady's test microscale experiment - student sheet
Editable handout | Word, Size 0.12 mbBrady's test microscale experiment - student sheet
Handout | PDF, Size 0.2 mbBrady's test microscale experiment - teacher notes
Editable handout | Word, Size 91.71 kbBrady's test microscale experiment - teacher notes
Handout | PDF, Size 0.21 mb
Additional information
This resource is part of our Microscale chemistry collection, which brings together smaller-scale experiments to engage your students and explore key chemical ideas. The resources originally appeared in the book Microscale chemistry: experiments in miniature, published by the Royal Society of Chemistry in 1998.
© Royal Society of Chemistry
Health and safety checked, 2018


















No comments yet