Observe the reactions of hydrogen sulfide with lead nitrate, silver nitrate and potassium manganate(VII) in this microscale practical
In this experiment, students react hydrogen sulfide with lead nitrate, silver nitrate and potassium manganate(VII) using microscale apparatus. They use a reaction vessel containing zinc sulfide and hydrochloric acid to generate the hydrogen sulfide gas inside a Petri dish, recording their observations as it reacts with the different test solutions.
The practical should take approximately 20 minutes.
Equipment
Apparatus
- Eye protection (goggles)
- Student information sheet and worksheet
- Clear plastic sheet (eg ohp sheet)
- Plastic Petri dish (base + lid), 9 cm diameter
- Plastic pipette
- Scissors
Chemicals
Note
Solutions should be contained in plastic pipettes – see the accompanying guidance on apparatus and techniques for microscale chemistry, which includes instructions on preparing solutions.
- Hydrochloric acid, 1 mol dm–3
- Lead nitrate, 0.5 mol dm–3
- Potassium manganate(VII), 0.01 mol dm–3
- Silver nitrate, 0.2 mol dm–3
- Sulfuric acid, 1 mol dm–3
- Zinc sulfide powder
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout (splash-resistant goggles to BS EN166 3) and work in a well-ventilated room.
- Hydrogen sulfide – see CLEAPSS Hazcard HC051A. Hydrogen sulfide is an extremely poisonous gas but because it can be detected by smell at very low concentrations it is much less dangerous than other gases such as carbon monoxide which, although less poisonous, cannot be detected by smell.
- Sulfuric acid – see CLEAPSS Hazcard HC098a and CLEAPSS Recipe Book RB098. At the concentration used, sulfuric acid is a skin/eye irritant.
- Lead nitrate, Pb(NO3)2(aq) – see CLEAPSS Hazcard HC057a and CLEAPSS Recipe Book RB053. At the concentration used, lead nitrate is a reproductive toxin, causes eye damage, causes damage to organs (especially the CNS) and is harmful to the aquatic environment. Avoid inhalation and skin contact.
- Silver nitrate – see CLEAPSS Hazcard HC087 and CLEAPSS Recipe Book RB077. At the concentration used, silver nitrate causes eye damage and is harmful to the aquatic environment.
- The following chemicals are of low hazard:
- Zinc sulfide – see CLEAPSS Hazcard HC108b.
- Hydrochloric acid – see CLEAPSS Hazcard HC047a and CLEAPSS Recipe Book RB043.
- Potassium manganate(VII), at the concentration used – see CLEAPSS Hazcard HC081 and CLEAPSS Recipe Book RB073.
Procedure
- Cover the worksheet with a clear plastic sheet.
- Place the base of the Petri dish directly over the circle below. Place the reaction vessel in the centre.
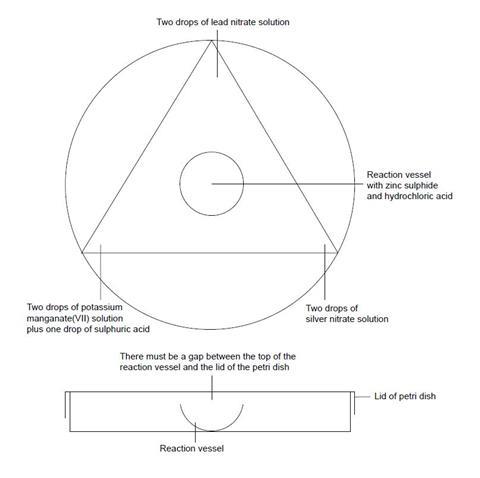
- At the corners of the triangle add drops of the test solutions as indicated in the diagram above.
- Add a small quantity of zinc sulfide powder to the reaction vessel followed by three drops of hydrochloric acid. Quickly replace the lid on the Petri dish.
- Record all your observations over the next 15 minutes. When you have finished add three drops of sodium hydroxide solution to the reaction vessel to stop the reaction.
Questions for students
- What explanations can you give for your observations?
- Why does sodium hydroxide stop the reaction?
Teaching notes
Method
Zinc sulfide + hydrochloric acid generates hydrogen sulfide:
ZnS(s) + 2HCl(aq) → ZnCl2 (s) + H2 S(g)
No bubbles of gas are seen and only small quantities of hydrogen sulfide are given off – sufficient, however, to carry out the tests.
Results
The lead nitrate solution gradually turns silvery due to the formation of lead sulfide by reaction of hydrogen sulfide and lead nitrate.
The silver nitrate solution reacts in a similar fashion although the reaction appears to be slower and the silver sulfide is less reflective than the lead sulfide.
The potassium manganate(VII) solution turns first brown and then, if sufficient gas is present, colourless, as it is reduced by the hydrogen sulfide:
2MnO4– (aq) + 5H2 S(g) + 6H+ (aq) → 5S(s) + 2Mn2+ (aq) + 8H2 O(l)
Downloads
Microscale reactions of hydrogen sulfide - student sheet
Editable handout | Word, Size 0.1 mbMicroscale reactions of hydrogen sulfide - student sheet
Handout | PDF, Size 0.18 mbMicroscale reactions of hydrogen sulfide - teacher notes
Editable handout | Word, Size 53.62 kbMicroscale reactions of hydrogen sulfide - teacher notes
Handout | PDF, Size 0.16 mb
Additional information
This resource is part of our Microscale chemistry collection, which brings together smaller-scale experiments to engage your students and explore key chemical ideas. The resources originally appeared in the book Microscale chemistry: experiments in miniature, published by the Royal Society of Chemistry in 1998.
© Royal Society of Chemistry
Health and safety checked, 2018


















No comments yet