Generate ethyne gas with calcium carbide and test its properties in this microscale class practical
In this experiment, students produce ethyne or acetylene gas inside a plastic Petri dish by reacting calcium carbide and water. The gas is tested using a solution of potassium manganate(VII) in propanone, which turns from purple to brown. When the reaction has finished, students test the residue of the calcium carbide with a drop of full-range indicator solution.
The practical should take approximately 20 minutes.
Find accompanying demonstrations
A version of this experiment at a larger scale, for demonstration purposes only, is available here. Also see the demonstration Incendiary silver for an impressive, linked, organometallic explosive.
Equipment
Apparatus
- Eye protection
- Clear plastic sheet (eg ohp sheet)
- Plastic pipettes
- Plastic Petri dish, 5.5 cm diameter (eg Philip Harris ref: Y 36340)
- Beaker, 10 cm3
- Scissors
- Tweezers
Chemicals
Note
Solutions and liquids should be contained in plastic pipettes. See the accompanying guidance on apparatus and techniques for microscale chemistry, which includes instructions for preparing a variety of solutions.
- Propanone
- Water
- Calcium carbide, small lumps
- Potassium manganate(VII) crystals
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout (splash-resistant goggles to BS EN166 3) and work in a well-ventilated area.
- Propanone is highly FLAMMABLE. See CLEAPSS Hazcard HC085A.
- Calcium carbide, CaC2(s), can produce flammable gasses when in contact with water, which may ignite spontaneously. Wear splash-proof goggles and ensure sufficient ventilation. See CLEAPSS Hazcard HC019C.
- Potassium manganate(VII) is an OXIDISER, harmful if swallowed and toxic to aquatic life. Avoid direct contact and store in the dark, stains glass, plastic, clothing and skin. See CLEAPSS Hazcard HC081.
Procedure
- Cover the worksheet with a clear plastic sheet.
- Place the base of the Petri dish over the large circle on the diagram (see below).
- Cut off the ends of two plastic pipettes (as shown below) and place them inside the Petri dish.
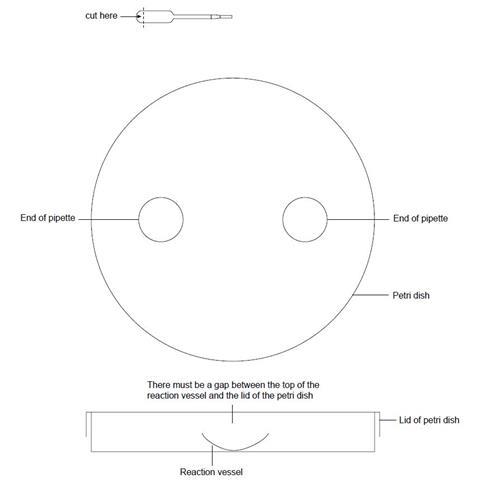
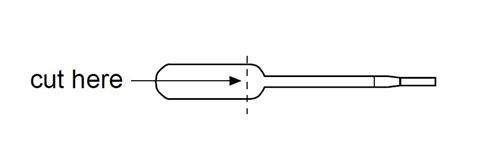
- Cut off the bulb of a plastic pipette as shown below and place it in a beaker.
- Carefully add a few crystals of potassium manganate(VII) to the pipette.
- Add propanone to the pipette until it is about half-full.
- Using a pipette, add four drops of the potassium manganate(VII) in propanone solution to one of the pipette ends in the Petri dish.
- Using tweezers carefully place one small lump of calcium carbide into the other pipette end.
- Carefully add four drops of deionised water to the calcium carbide and quickly place the lid on the Petri dish.
- Observe any changes over the next few minutes.
- When no more gas is formed add one drop of full-range indicator solution to the residue of the calcium carbide and observe.
Teaching notes and expected observations
Calcium carbide reacts vigorously with water. The ethyne gas produced turns potassium manganate(VII) from purple to brown indicating ethyne is unsaturated. The residue turns full-range indicator solution purple owing to the presence of the alkaline calcium hydroxide.
This experiment illustrates an interesting link between inorganic and organic chemistry. However, calcium carbide is an expensive material. It is prepared by heating a mixture of calcium oxide and coke to around 2000 °C in an electric furnace.
Students could also be told about Wohler’s experiment in 1828 on heating ammonium cyanate and obtaining urea thus demolishing the vital force theory (that organic compounds could only be obtained from living things).
Downloads
Preparing and testing ethyne (acetylene) - student sheet
Editable handout | Word, Size 0.1 mbPreparing and testing ethyne (acetylene) - student sheet
Handout | PDF, Size 0.23 mbPreparing and testing ethyne (acetylene) - teacher notes
Editable handout | Word, Size 50.9 kbPreparing and testing ethyne (acetylene) - teacher notes
Handout | PDF, Size 0.13 mb
Additional information
This resource is part of our Microscale chemistry collection, which brings together smaller-scale experiments to engage your students and explore key chemical ideas. The resources originally appeared in the book Microscale chemistry: experiments in miniature, published by the Royal Society of Chemistry in 1998.
© Royal Society of Chemistry
Health and safety checked, 2018


















No comments yet