Use a solution of potassium manganate to test for unsaturation in organic compounds in this microscale practical
In this experiment, students use a solution of potassium manganate(VII) in propanone to detect whether an organic compound is unsaturated. The propanone solution is made up and stored in a plastic pipette, and mixes easily with non-polar organic compounds such as cyclohexane, cyclohexene and limonene. Unsaturated compounds will turn the solution a brownish colour as the manganese(VII) is reduced to manganese(IV).
The practical should take approximately 20 minutes.
Equipment
Apparatus
- Eye protection
- Plastic pipettes
- Plastic Petri dish
- Beaker, 10 cm3
- Scissors
Chemicals
- Propanone
- Potassium manganate(VII) crystals
- Cyclohexane
- Cyclohexene
- Limonene
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Avoid sources of ignition.
- Propanone is HIGHLY FLAMMABLE. See CLEAPSS Hazcard HC085A.
- Potassium manganate(VII) is an OXIDISER and IRRITANT, and stains glass, plastic, clothing and skin. Avoid direct contact and store in the dark. See CLEAPSS Hazcard HC081.
- Cyclohexane is HIGHLY FLAMMABLE, a skin/respiratory IRRITANT and very TOXIC to aquatic life. See CLEAPSS Hazcard HC045b.
- Cyclohexene is a HIGHLY FLAMMABLE liquid and vapour. It is HARMFUL if ingested or in contact with the skin and is very TOXIC to aquatic life. See CLEAPSS Hazcard HC045c.
- Limonene is FLAMMABLE as liquid and vapour. It is TOXIC to aquatic life and is an IRRITANT and SENSITISER to skin. See CLEAPSS Hazcard HC045c.
Procedure
- Cut the end off a plastic pipette as shown below and place the cup in a glass beaker or test tube.
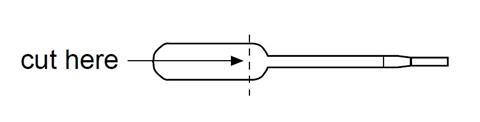
- Carefully add a few crystals of potassium manganate(VII) to the cup.
- Add propanone to the cup until it is about half-full. You will notice that the potassium manganate(VII) dissolves to give a purple solution. Is it surprising that potassium manganate(VII) dissolves in an organic solvent?
- Cut the ends off three pipettes to make small reaction vessels as shown below and place them in the lid of a plastic Petri dish.
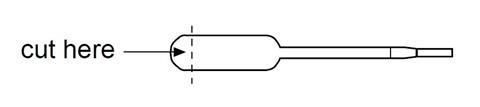
- Using a plastic pipette, add four drops of the potassium manganate(VII) in propanone solution to each of the reaction vessels.
- Put three drops of each of the organic liquids under test in the reaction vessel and observe any changes over the next few minutes.
- Mop up the liquid with tissue paper when you have finished.
Question for students
Which types of organic liquids react with potassium manganate(VII)?
Downloads
Unsaturation test with potassium manganate - student sheet
Editable handout | Word, Size 60.29 kbUnsaturation test with potassium manganate - student sheet
Handout | PDF, Size 0.14 mbUnsaturation test with potassium manganate - teacher notes
Editable handout | Word, Size 61.61 kbUnsaturation test with potassium manganate - teacher notes
Handout | PDF, Size 0.14 mb
Additional information
This resource is part of our Microscale chemistry collection, which brings together smaller-scale experiments to engage your students and explore key chemical ideas. The resources originally appeared in the book Microscale chemistry: experiments in miniature, published by the Royal Society of Chemistry in 1998.
© Royal Society of Chemistry
Health and safety checked, 2018


















No comments yet