Help your 16–18 students tackle pH, buffer solutions and the Henderson–Hasselbalch equation with this infographic and accompanying worksheet
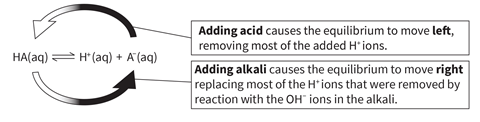
Changes in pH can have a big impact on natural systems, so it’s important that the hydrogen ion concentration stays relatively constant. Buffers minimise the change in pH that would otherwise happen if an acid or alkali were added to an aqueous solution.
A buffer is created when a weak acid and its salt are both present in solution. The buffer solution utilises the reversible reaction involving the loss of a proton from the weak acid.
Download this
This infographic is designed to be displayed as a poster in the classroom, although it can also be displayed on a projector or printed as a handout.
Use the accompanying fact sheet and worksheet to get your students to apply their mathematical skills in different contexts.
- Poster as pdf (A4 single pages and A3 one page)
- Fact sheet as MS Word or pdf
- Teacher notes and answers as MS Word or pdf
- Student worksheet as MS Word or pdf
The pH of a buffer solution can be calculated using the Henderson–Hasselbalch equation, which is derived from the equilibrium constant equation for dissociation of a weak acid with general formula HA.
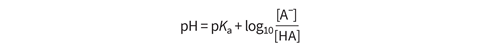
Covid-19 lateral flow test
The lateral flow test for Covid-19 contains antibodies to a protein that is produced by the virus. The binding of the antibody to the virus protein will only work if the pH is approximately 7.4.
The sample taken from the nose is mixed with a phosphate buffer solution to prevent any acids or alkalis in the sample from changing the pH too much.
- H2PO4-(aq) ⇌ H+(aq) + HPO42-(aq) pKa = 7.21 at 25°C

Healthy hair
The ideal pH for the scalp is around 5.5. Shampoo ingredients are often more alkaline than this, which could damage the scalp and change the properties of the hair.
In a pH balanced shampoo, a buffer is added to keep the pH at 5.5 or lower. Citric acid is often used as it can control the pH at any value from 3 to 7.
- Dissociation of the first H+ ion: C6H8O7(aq) ⇌ H+(aq) + C6H7O7-(aq) pKa = 3.13 at 25°C
- Dissociation of the second H+ ion: C6H7O7-(aq) ⇌ H+(aq) + C6H6O72-(aq) pKa = 4.76 at 25°C
- Dissociation of the third H+ ion: C6H6O72-(aq) ⇌ H+(aq) + C6H5O73-(aq) pKa = 6.39 at 25°C

Oceans in peril
The increased level of atmospheric carbon dioxide due to human activity is leading to a decrease in pH of the oceans. This is affecting marine ecosystems, in particular the survival of plankton, molluscs and coral that depend on dissolved carbonate ions to make their shells.
Dissolved carbon dioxide increases the ratio of hydrogen carbonate to carbonate in the natural buffer system that helps control ocean acidity.
- HCO3-(aq) ⇌ H+(aq) + CO32-(aq) pKa = 10.32 at 25°C
Put this in context
Discover how senior scientist, Phillip, works with a team of researchers looking for new ways to improve the performance of household products such as toothpaste, nappies, makeup and shampoo.
All illustrations © Dan Bright
All equations © Royal Society of Chemistry
Downloads
Brilliant buffers infographic poster A4 single pages
Handout | PDF, Size 0.41 mbBrilliant buffers infographic poster A3 one page
Handout | PDF, Size 0.98 mbBrilliant buffers fact sheet
Editable handout | Word, Size 0.1 mbBrilliant buffers fact sheet
Handout | PDF, Size 0.16 mbBrilliant buffers teacher notes
Editable handout | Word, Size 90.1 kbBrilliant buffers teacher notes
Handout | PDF, Size 0.21 mbBrilliant buffers student worksheet
Editable handout | Word, Size 0.12 mbBrilliant buffers student worksheet
Handout | PDF, Size 0.2 mb













1 Reader's comment