All Physical chemistry articles – Page 10
-
 Feature
FeatureTeaching chemistry in 3D using crystal structure data
Fundamental topics such as stereochemistry are taught in 2 or 2.5D - the Cambridge Structural Database provides an interactive 3D solution
-
 Exhibition chemistry
Exhibition chemistryFloating Gas Bubbles
Demonstrations designed to capture the student's imagination
-
 Feature
FeatureChemical Bonding
A masterclass in teaching the topic of bonding, basing chemical explanation on physical forces
-
 Feature
FeatureInvestigating Crystal Structures
Sixthformers are introduced to Madelung constants as a way of investigating ionic crystal structures
-
 Feature
FeatureWhat is entropy?
What's the best way to introduce to your students this most misunderstood of thermodynamic properties?
-
 Lesson plan
Lesson planReacting iron and sulfur to explore compounds | 11-14 years
Investigate the reaction between iron and sulfur and practise modelling chemical changes in this lesson plan with activities for 11–14 year olds.
-
 Lesson plan
Lesson planWhat is stuff made of? Matter, atoms and elements | 11-14 years
Introduce the properties and behaviour of atoms as the smallest parts of elements and a basic unit of matter using this lesson plan for 11–14 year olds.
-
 Lesson plan
Lesson planConservation of mass in dissolving and precipitation | 11-14 years
Explore what happens during precipitation reactions and when substances dissolve using this lesson plan with downloadable activities for 11–14 year olds.
-
 Lesson plan
Lesson planWhat happens when a substance changes state? | 11-14 years
Use this lesson plan for 11–14 year olds to explore what happens when substances warm, cool, boil or freeze, tackling misconceptions about changes of state.
-
 Lesson plan
Lesson planParticle models: gas, liquid, solid | 11-14 years
Help your students develop their understanding of gases, liquids and solids using the particle model in this lesson plan with activities for 11–14 year olds.
-
 Lesson plan
Lesson planWhat happens when something dissolves? | 11-14 years
Explore the process of dissolving and help your students explain observations using the particle model with this lesson plan and activities for 11–14 year olds.
-
 Lesson plan
Lesson planWhat properties do gases have? | 16-18 years
Investigate the properties of gases and address common misconceptions among students using this lesson plan and series of small experiments for 16–18 year olds.
-
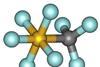 Feature
FeatureCF3SF5 - a 'super' greenhouse gas
Trifluoromethyl sulfur pentafluoride - a byproduct of the electronics industry - has been named a 'super' greenhouse gas by physical chemists
-
 Resource
ResourceAtoms and nanochemistry
From practical experiments to model-building and presentations, discover activities to help 11–16 year olds learn about atoms, atomic imaging and nanochemistry.
-
 Feature
FeatureMaking ice cream - it's physical chemistry
An understanding of the physical chemistry of ice cream is the route to a smooth, soft, creamy dessert











