All Resource articles – Page 53
-
 Resource
ResourceChemistry Olympiad support booklet
Stretch and challenge your students or help them prepare for the Chemistry Olympiad using these example questions with commentary and analysis.
-
 Resource
ResourceChemistry stinks - olfactory indicators experiment
Chemicals that change colour with pH are frequently used as indictors for acid-base titrations. The aim of this experiment it to determine the concentration of an unknown base using garlic powder as an olfactory indicator. Chemistry stinks – olfactory indicators experiment Class practical Chemicals that change colour with pH ...
-
 Resource
ResourceChemistry stinks - class smelling activity
This class practical introduces concepts of chemistry and smell. A number of common chemicals with distinctive smells have been chosen to help students get their nose around different functionalities in chemistry. This is done via a series of solutions, which can be smelled using smelling sticks.
-
 Resource
ResourceMolymod sets
These popular molecular modelling sets can be used to make many different molecules. This makes them ideal for use by students, educators and researchers. Follow the links below buy the sets in our online shop. If you’re a member of the Royal Society of Chemistry, you’re entitled to a 35% ...
-
 Resource
ResourceThe greenhouse effect and global warming
An activity worksheet to test student understanding of global warming.
-
 Resource
ResourceLooking at climatic data from the past
Data analysis worksheet looking at ocean sediment and water temperatures.
-

-
 Resource
ResourceSolar Fuels and Artificial Photosynthesis
A variety of infographics (diagrams) explaining photosynthesis, artificial photosynthesis, and the production and uses of solar fuels.
-
 Resource
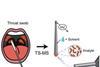
ResourceJournal articles made easy: Detect strep throat
This article looks at detecting strep throat bacterium using touch spray mass spectrometry. It will help you understand the research the journal article is based on, and how to read and understand journal articles. The research article was originally published in our Analyst journal. Strep throat spotted in secondsDetection of ...
-
 Resource
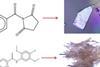
ResourceJournal articles made easy: Predict crystallinity
This article looks at predicting and controlling the crystallinity of molecular materials. It will help you understand the research the journal article is based on, and how to read and understand journal articles. The research article was originally published in our CrystEngComm journal. Will it crystallise?Predicting crystallinity of molecular materialsClick ...
-
 Resource
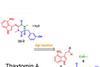
ResourceJournal articles made easy: A natural herbicide
This article looks at developing a natural product herbicide, thaxtomin A. It will help you understand the research the journal article is based on, and how to read and understand journal articles. The research article was originally published in our Organic & Biomolecular Chemistry journal.Natural product herbicide (±)-thaxtomin AA one-pot ...
-
 Resource
ResourceJournal articles made easy: Detecting iron
This article looks at measuring iron in a variety of solutions using a scalable RGB technique. It will help you understand the research the journal article is based on, and how to read and understand journal articles. The research article was orginally published in Journal of Materials Chemistry A. Detecting ...
-
 Resource
ResourceOther greenhouse gases
Increasing levels of carbon dioxide in the atmosphere cause a rise in global temperature. This worksheet looks at some data from other greenhouse gases to see if they have the same effect.
-
 Resource
ResourceTheories about global warming
This worksheet is intended to introduce theories about the causes of global warming. Use to initiate a class discussion, find out what the class already know about global warming and if they have any strong views on the subject.
-
 Resource
ResourceMario Molina puts ozone on the political agenda
A set of resources on Mario Molina’s work on ozone and its impact on global politics.
-
 Resource
ResourceModern applications
Inspirational chemistry book chapter ‘Modern applications’. This chapter suggests games, activities, practicals and demonstrations for teaching various topics, such as observing reactions, making new medicines and composite materials.
-
 Resource
ResourceLarge molecules
The Inspirational chemistry chapter ‘Large molecules’. This chapter suggests games, activities, practicals and demonstrations for teaching various topics, such as polmers, emulsifiers and textile conservation.
-
 Resource
ResourceProblem based practical activities
Discover how chemistry can relate to real world problems, so students can put their science knowledge into context.
-
 Resource
ResourceInspirational chemistry book
A collection of resources, aligned with GCSE bodies, to support learners in England, Wales, and N Ireland.
-
 Resource
ResourceWhat use is chemistry?
The Inspirational chemistry chapter ‘What use is chemistry?’ This chapter suggests exercises for students to carry out to appreciate the importance of chemistry and how it affects our everyday lives.











