Explore understanding and check for common misconceptions about ionic bonding
Use these 20 true or false questions to clarify understanding of ionic bonding using the example of sodium chloride.
-

Download this
A true or false worksheet with teacher guidance and answers to help identify and address any misconceptions learners may hold about ionic bonding.
View and download more Chemical misconceptions resources
How to use this resource
Use the student sheet to identify and address any misconceptions about ionic bonding. Some learners see ionic bonding in sodium chloride as a molecular phenomenon, with discrete pairs which are internally ionically bonded but attracted to each other by weaker forces (read more about learners’ misconceptions about chemical bonding).
- When to use? Use with learners in the 14–16 age range who have studied chemical bonding to develop, revise or assess the topic.
- Group size? Suitable for independent work either in class or at home. Or use the questions for group or class discussions to diagnose learners’ misconceptions.
- How long? 10–15 mins
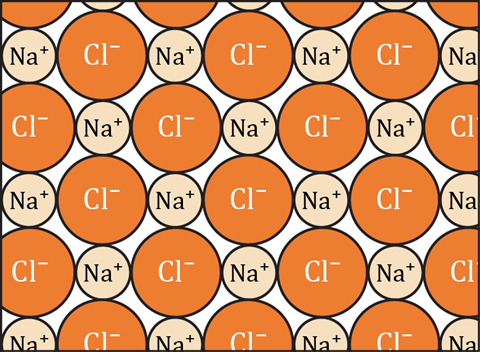
Emphasise to learners that the diagram shows just a small part of a slice through the lattice structure and that the real structure is three-dimensional.
Provide learners with a copy of the answers after they have completed the student sheet.
Answers
- A positive ion will be attracted to any negative ion.
TRUE: Any positively charged object will be attracted to any negatively charged object. It does not matter how the objects acquired the charge; the attraction only depends on the amount of charge and the distance between the two charged objects. - A sodium ion is only bonded to the chloride ion it donated its electron to.
FALSE: Each positive sodium ion is bonded to each of the neighbouring negative chloride ions. It is irrelevant how the ions came to be charged. - A sodium atom can only form one ionic bond because it only has one electron in its outer shell to donate.
FALSE: A sodium ion can strongly bond to as many chloride ions as can effectively pack around it in the regular crystal lattice. In NaCl there will be six chloride ions strongly bonded to each sodium ion. - A bond is formed between chloride ions and sodium ions because an electron has been transferred between them.
FALSE: The reason a bond is formed between chloride ions and sodium ions is because they have opposite electrostatic charges - negative and positive. - In the diagram, a chloride ion is attracted to one sodium ion by a bond and is attracted to other sodium ions just by forces.
FALSE: In the diagram each chloride ion is attracted to up to four sodium ions by a bond that is an electrostatic force. (There would also be a fifth sodium ion above the chlorine ion and one more below - but these are not shown in the diagram.) - In the diagram, each molecule of sodium chloride contains one sodium ion and one chloride ion.
FALSE: There are no molecules in sodium chloride, just ions. A molecule is comprised of a group of atoms strongly bound together, and only weakly bonded (if at all) to other molecules. In sodium chloride, each ion is strongly bonded to each of its six nearest neighbours. - An ionic bond is the attraction between a positive ion and a negative ion.
TRUE: An ionic bond is the attraction between a positive ion and a negative ion. - A positive ion can be bonded to any neighbouring negative ions, if it is close enough.
TRUE. The bond is just the attraction between the oppositely charged ions. If the ions are close together this force will be a strong bond. - A negative ion can be attracted to any positive ion.
TRUE: Any negatively charged object will be attracted to any positively charged object. It does not matter how the objects acquired the charge, the attraction only depends on the amount of charge and the distance between the two charged objects. - You cannot identify ionic bonds, unless you know which chloride ions accepted electrons from which sodium ions.
FALSE: As the bonding is just the attraction between ions, there will be a bond between any adjacent oppositely charged ions. - A chloride ion is only bonded to the sodium ion it accepted an electron from.
FALSE: Each negative chloride ion is bonded to each of the neighbouring positive sodium counter-ions. It is irrelevant how the ions came to be charged. - A chlorine atom can only form one ionic bond, because it can only accept one more electron into its outer shell.
FALSE: A chloride ion can strongly bond to as many sodium ions as can effectively pack around it in the regular crystal lattice. In NaCl there will be six sodium ions strongly bonded to each chloride ion. - There is a bond between the ions in each molecule, but no bonds between the molecules.
FALSE: There are no molecules in sodium chloride, but a continuous network of bonds throughout the lattice. - A negative ion can only be attracted to one positive ion.
FALSE: There is no limit to the number of positive ions that a negative ion can be attracted to (although there is a limit to how many can cluster around it). - A bond is formed between chloride ions and sodium ions because they have opposite charges.
TRUE: The opposite charges attract them together, and this electrostatic force of attraction is the ionic bond. - In the diagram, a sodium ion is attracted to one chloride ion by a bond and is attracted to other chloride ions just by forces.
FALSE: In the diagram, each sodium ion is attracted to up to four chloride ions by a bond that is an electrostatic force. (There would also be a fifth chloride ion above the sodium ion and one more (a sixth) below - but these are not shown in the diagram.) - A positive ion can only be attracted to one negative ion.
FALSE: There is no limit to the number of positive ions that a negative ion can be attracted to (although there is a limit to how many can cluster around it). - An ionic bond is when one atom donates an electron to another atom, so that they both have full outer shells.
FALSE: An ionic bond is the electrostatic force which holds two oppositely charged ions together. The ions could have become charged by electron transfer, but usually the ions were charged long before they came into contact. - A negative ion can be bonded to any neighbouring positive ions if it is close enough.
TRUE. The bond is just the attraction between the oppositely charged ions. If the ions are close together this force will be a strong bond. - There are no molecules shown in the diagram.
TRUE: A molecule is comprised a group of atoms strongly bound together, and only weakly bonded (if at all) to other molecules. In sodium chloride, each ion is strongly bonded to each of its six nearest neighbours.
Downloads
Ionic bonding true or false student sheet
Handout | PDF, Size 0.21 mbIonic bonding true or false teacher guidance
Handout | PDF, Size 0.28 mbIonic bonding true or false student sheet
Editable handout | Word, Size 0.52 mbIonic bonding true or false teacher guidance
Editable handout | Word, Size 0.57 mb
Websites
Additional information
These resources have been taken from the book Chemical Misconceptions: Prevention, Diagnosis and Cure: Theoretical Background, Volume 2, by Keith Taber.
The resource was adapted and updated in 2025 by Kirsty Patterson.

Chemical misconceptions

Discover classroom strategies and activities to tackle common misconceptions among students in chemistry, and explore the theory behind different approaches.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
 Currently
reading
Currently
reading
Ionic bonding: true or false?
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36







































































































1 Reader's comment