Try this class practical to make a plastic using potato starch
In this activity, students use potato starch to produce a plastic. Three versions of the method are offered: a non-lab version that can be carried out in a range of contexts, a simple lab-based version using household ingredients and the original experiment using dilute hydrochloric acid and sodium hydroxide. Learners can go on to investigate how adding a ‘plasticiser’ affects the properties of their polymer.
Students can begin either with potatoes or with commercially bought potato starch. The practical is straightforward, the main hazard being the mixture boiling dry.
If you begin with potatoes, extracting the starch takes about 15–20 minutes and making the plastic film about 20 minutes. A large pestle and mortar is needed to extract enough starch to make sufficient plastic to cover a petri dish. If these are not available, students can use a smaller one and top up the starch they extract with commercially available potato starch.
Simple experiment using household ingredients
Equipment and preparation
Depending on the size of the group and the amount of equipment to hand, the practical takes 10–25 minutes to complete and is suitable to be carried out in pairs or threes. The plastic takes roughly seven days to dry, therefore it is recommended that you try the practical beforehand and take in some pre-prepared samples for discussion.
All the ingredients can be purchased from supermarkets or online. The experiment can be carried out in a kitchen, classroom (with adequate heating facilities), home economics space or laboratory.
You will need:
- Water
- Potato starch
- Vegetable glycerine (also known as glycerin or glycerol)
- Vinegar
- Teaspoon or access to a laboratory balance
- Tablespoon or access to measuring cylinders
- Bowl or beaker
- Spatula or glass rod
- Pan or beaker
- Food colouring (optional)
- Stove or stirrer hotplate
- Greaseproof paper
- Foil
- Safety glasses or goggles
Method
If you are in a laboratory, you can use glassware, balances and measuring cylinders. If you are not, you can use baking measures. Both methods are listed below.
Non-laboratory setting
1. Take 1 level tablespoon of potato starch and place in a bowl.
2. Add 7 tablespoons of water to the bowl and stir.
3. Add 2 teaspoons of vinegar to the bowl.
4. Add 2 teaspoons of glycerine to the bowl and stir.
5. (Optional – add 2 drops of food colouring and stir).
6. Pour the mixture into a pan, put it on the stove on a medium-high heat and stir continually.
7. Stir with a plastic spatula until the mixture thickens. Do not let the mixture boil dry.
8. When jelly-like, pour onto greaseproof paper with foil underneath.
9. Flatten with a spatula to roughly 8 inches in diameter, taking care to make the thickness even all over.
Top tips
- Practise making the plastic before the session. Show all the batches you make to the learners after the experiment to demonstrate what theirs will look like when it’s dry, even ones that didn’t work as well as you were expecting.
- Consider doing the experiment along with the learners. Get them all to complete each step before moving onto the next one. This means no one gets left behind and everyone understands the instructions you’re giving them.
- Do not let the mixture boil dry; it ‘pops’ and tends to jump out of the pan or beaker. This is why everyone doing this experiment should wear eye protection.
- You can substitute corn starch for potato starch, but the resulting plastic is less robust, more opaque and tends to break apart.
- Watch our Tiktok video and share it with learners, to preview or recap the experiment and the chemistry that lies behind it.
Laboratory setting
1. Take 15 g of potato starch and place in a beaker.
2. Add 100 cm3 of water to the beaker and stir.
3. Add 10 cm3 of vinegar to the beaker.
4. Add 10 cm3 of glycerine to the beaker and stir.
5. (Optional – add 2 drops of food colouring and stir).
6. Place the beaker on a hotplate (or equivalent heating method, depending on availability) on a medium-high heat and stir continually.
7. Stir with a glass rod until the mixture thickens. Do not let the mixture boil dry.
8. When jelly-like, pour onto greaseproof paper with foil underneath.
9. Flatten with a spatula to roughly 8 inches in diameter, taking care to make the thickness even all over.
Leave the plastic to dry for at least 7 days. Do not leave it anywhere exposed to extremes of heat, or it will likely crack.
Safety and hazards
Read our standard health and safety guidance and carry out a risk assessment before running any practical.
- Wear eye protection (safety glasses or goggles) throughout.
- The hotplate or pan is a burn risk.
- The mixture becomes very hot and is a burn risk.
- Glass beakers and glass rods are a burn and shatter risk.
- Use minimum quantities of chemicals.
- Potato plastic should not be eaten.
- Dispose of potato plastic in compostable waste.
Background chemistry and questions
Find discussion prompts and notes on the background chemistry in the Teaching notes. Use the Curriculum links to help you understand how to connect with learners level of scientific understanding.
More resources
- Explore plant-based plastics further with this reading and talking heads activity.
- Reinforce knowledge and understanding of recycling and life cycle assessments with this recycling plastics evaluation.
- Introduce learners to some real science research, from using plants to recycle polythene to turning plastic bottles into jet fuel.
- Use our video job profiles to connect this topic to science careers, for example Celine develops ways to alter plastics to help them biodegrade.
Full laboratory method
The original method for this practical, including an investigation into how adding a ‘plasticiser’ affects the properties of their polymer.
Equipment and preparation
- Eye protection
- Grater
- Beakers, 400 cm3, x4
- Large pestle and mortar
- Beaker, 250 cm3
- Large watch glass
- Bunsen burner
- Heat resistant mat
- Tripod
- Gauze
- Stirring rod
- Petri dish or white tile
- Universal indicator paper
- Teat pipettes
- Measuring cylinder, 25 cm3
- Measuring cylinder, 10 cm3
- Access to a tea strainer
- Access to a balance (optional – see note 5 below)
Chemicals
- Dilute hydrochloric acid, 0.1 M, about 10 cm3
- Dilute sodium hydroxide, 0.1 M (IRRITANT), about 10 cm3
- Potatoes, 100 g
- Distilled water, about 500 cm3
- Access to:
- Potato starch, either extracted in the first part or 2.5 g of bought potato starch (see note 6 below)
- Food colouring
- Propane-1,2,3-triol (glycerol), 2 cm3
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Dilute hydrochloric acid, HCl(aq) – see CLEAPSS Hazcard HC047a and CLEAPSS Recipe Book RB043.
- Dilute sodium hydroxide, NaOH(aq), (IRRITANT at this concentration) – see CLEAPSS Hazcard HC091a and CLEAPSS Recipe Book RB085.
- If access to a balance is difficult get students to use a heaped spatula of starch rather than 2.5 g.
- Even if students are extracting their own starch, it is worth having some commercial potato starch available in case they do not extract enough.
- If a drying cabinet is available, it is useful for drying out the plastic films. It takes about 90 mins at 100°C.
Procedure
Extracting the starch
- Grate about 100 g of potato. The potato does not need to be peeled, but it should be clean. Put the potato into the mortar.
- Add about 100 cm3 of distilled water to the mortar, and grind the potato carefully.
- Pour the liquid off through the tea strainer into the beaker, leaving the potato behind in the mortar.
- Repeat steps 2 and 3 twice more.
- Leave the mixture to settle in the beaker for five minutes.
- Decant the water from the beaker, leaving behind the white starch which should have settled in the bottom. Put about 100 cm3 of distilled water in with the starch and stir gently. Leave to settle again and then decant the water, leaving the starch behind.
Making the plastic film
- Put 22 cm3 of water into the beaker and add 4 g of the potato starch slurry from the previous step (or 25 cm3 water and 2.5 g of commercial potato starch), 3 cm3 of hydrochloric acid and 2 cm3 of propane-1,2,3-triol.
- Put the watch glass on the beaker and heat the mixture using the Bunsen burner. Bring it carefully to the boil and then boil it gently for 15 mins. Do not boil it dry. If it looks like it might, stop heating.
- Dip the glass rod into the mixture and dot it onto the indicator paper to measure the pH. Add enough sodium hydroxide solution to neutralise the mixture, testing after each addition with indicator paper. You will probably need to add about the same amount as you did of acid at the beginning (3 cm3).
- You can then add a drop of food colouring and mix thoroughly.
- Pour the mixture onto a labelled petri dish or white tile and push it around with the glass rod so that there is an even covering.
- Repeat the process, but leave out the propane-1,2,3-triol.
- Label the mixtures and leave them to dry out. It takes about one day on a radiator or sunny windowsill, or two days at room temperature. Alternatively, use a drying cabinet. It takes about 90 mins at 100°C.
Teaching notes
This activity can be used simply as a practical to enhance the teaching of polymers or plastics. It can be used to introduce further work on biopolymers and bioplastics and/or it can be used as an example of the effects of plasticisers. A similar process is used in industry to extract starch, which is then used in a number of products including food and packaging.
If students extract their own potato starch then they can use that. It is a wet slurry rather than a dry powder so they need about 4 g with about 22 cm3 water. If they do not have enough then they can add a bit of bought potato starch to the mix.
If access to a balance is difficult, then get students to use a heaped spatula of starch rather than 2.5 g.
If access to 10 cm3 measuring cylinders is difficult, then get students to use four pipette squirts of hydrochloric acid and three squirts of propane-1,2,3-triol.
If you have a drying cabinet, the mixture should dry in about 90 mins at 100°C.
Warn students not to let the mixture boil dry, or it ‘pops’ and has a tendency to jump out of the beaker. For this reason, students should wear eye protection at all stages.
While using food colouring is optional, it does enhance the product and the colour it gives makes the plastic film look more like plastic. Only one drop is needed or the film is too dark.
If students use too much water then their polymer won’t solidify and remains a liquid.
Starch is made of long chains of glucose molecules joined together. Strictly it contains two polymers: amylose which is straight-chained and amylopectin which is branched. When starch is dried from an aqueous solution it forms a film due to hydrogen bonding between the chains. However, the amylopectin inhibits the formation of the film. Reacting the starch with hydrochloric acid breaks down the amylopectin, forming more satisfactory film. This is the product that students make without propane-1,2,3-triol. The straight chains of the starch (amylose) can line up together and although this makes a good film, it is brittle because the chains are too good at lining up. Areas of the film can become crystalline, which causes the brittleness.
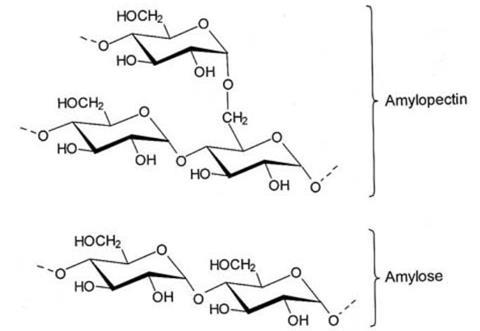
Students should be able to see a difference in the two films that they make. The one without the propane-1,2,3-triol is far more brittle, the one with it shows more plastic properties.
Adding propane-1,2,3-triol makes a difference due to its hydroscopic (water attracting) properties. Water bound to the propane-1,2,3-triol gets in amongst the starch chains and stops the crystalline areas from forming, preventing the brittleness and resulting in more ‘plastic’ properties, thus acting as a plasticiser. This can be explained to students without mentioning water – just that the propane-1,2,3-triol acts as a plasticiser.
Downloads
Making plastic from potato starch teaching notes
Handout | PDF, Size 0.35 mbMaking plastic from potato starch presentation
Presentation | PDF, Size 0.92 mbMaking plastic from potato starch curriculum links
Handout | PDF, Size 0.34 mbMaking plastic from potato starch presentation
Presentation | PowerPoint, Size 3.69 mbMaking plastic from potato starch teaching notes
Editable handout | Word, Size 0.48 mb
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry
This resource was updated in 2024 to add the simple version using household ingredients and additional downloadable resources, written by Joanna Buckley.






















2 readers' comments