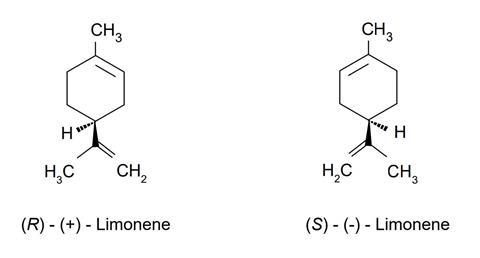
Uncover the diversity of limonene in this experiment, where students explore two enantiomers of this distinctively smelling chemical
When life gives you lemons … sniff them! This experiment will see students discovering the differences between two different enantiomers of limonene, using their scientific curiosity, and their noses.
This experiment should take 5 minutes.
Equipment
Apparatus
- Plastic bottles x2
- Cotton wool
Chemicals
- (R)-(+)-Limonene
- (S)-(–)-Limonene
Health, safety and technical notes
- Read our standard health and safety guidance.
- Students must wear eye protection if carrying out steam distillation.
- Not needed for sniffing the stereoisomers.
Procedure
In this experiment, students detect the differences in smell of each enantiomer absorbed on cotton wool inside small sample bottles.
To prepare these;
- Place a small quantity of cotton wool into each bottle.
- Add 10 drops of the stereoisomer.
- The bottles can then be passed around the classroom.
Questions
Would you expect the two stereoisomers of limonene to behave differently in their:
- Reaction with bromine?
- Reduction with hydrogen?
- Melting point?
- Boiling point?
- Infrared spectrum?
- Effect on plane of polarisation of plane-polarised light?
- Combustion?
- Mass spectrum?
Explain your answers.
Extension
Students could obtain small quantities of (R)-(+)- limonene in natural fruits by carrying out steam distillation of the peel of citrus fruits such as oranges and lemons and comparing the odours against the standards.
However, the S-(–) isomer is scarce in citrus fruits: pine needles might be a good source, but the presence of other terpenes might make it hard to separate.
Downloads
Properties of stereoisomers - teacher notes
PDF, Size 0.16 mbProperties of stereoisomers - teacher notes
Word, Size 97.55 kbProperties of stereoisomers - student sheet
PDF, Size 0.16 mbProperties of stereoisomers - student sheet
Word, Size 96.52 kb
References
S. W. Breuer, Microscale practical organic chemistry. Lancaster: Lancaster University, 1991.
Additional information
This resource is part of our Microscale chemistry collection, which brings together smaller-scale experiments to engage your students and explore key chemical ideas. The resources originally appeared in the book Microscale chemistry: experiments in miniature, published by the Royal Society of Chemistry in 1998.
© Royal Society of Chemistry
Health and safety checked, 2018

















No comments yet