Test the melting points of lead, tin and solder to investigate solder as a solid mixture and alloy in this practical
Electric solder is an alloy of tin with one or more other metals. Tin–lead solders were commonly available but now lead-free solders are used for manufacturing, and lead based solders are becoming increasingly difficult to obtain.
In this experiment, students heat samples of tin, lead and tin–lead solder to compare their melting points, observing that the metal alloy has a much lower melting point than either of the pure metals. It shows how such an alloy is more convenient and safer to work with when soldering.
The experiment is conveniently carried out by groups of two and will take about 30 minutes.
Equipment
Apparatus
- Eye protection
- Bunsen burner
- Tripod
- Heat resistant mat
- Pipeclay triangle
- Crucible lid
Chemicals
- Tin, a small piece
- Lead (TOXIC, DANGEROUS FOR THE ENVIRONMENT), a small piece
- Flux-free solder, a small piece
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout. Be very careful to avoid coming into contact with molten drops of metal. Ensure good ventilation. It may be advisable for asthmatic students to work in a fume cupboard.
- Tin, Sn(s) – see CLEAPSS Hazcard HC102A.
- Lead, Pb(s), (TOXIC, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC056.
- Flux-free solder – it is important the solder is flux-free. Fumes produced when solder containing rosin-based flux is used, can irritate the respiratory system and in some cases has caused sensitisation.
Procedure
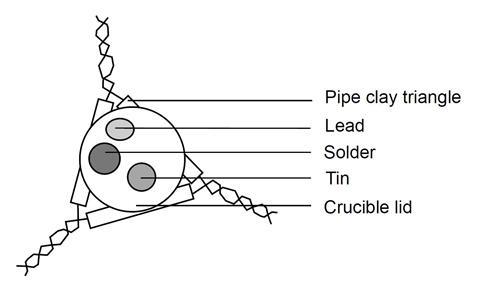
- Place a small piece of tin, lead and solder on an inverted crucible lid. Make sure you know which lump is which!
- Place the crucible lid on a pipe clay triangle, on a tripod. Place a lighted Bunsen burner underneath, on a heat resistant mat, and heat the lid gently.
- Observe the three lumps to see the order in which they melt.
- When all three are molten, turn off the Bunsen burner and allow everything to cool.
- Note the order in which the lumps solidify again.
Teaching notes
Remind students of the dangers of coming into contact with hot molten metal.
Good laboratory ventilation is important, particularly if a large number of experiments are being carried out. Asthmatics should be asked to carry out their experiments using a fume cupboard.
A common problem with this experiment is that students forget which lump is which.
The melting points of tin and lead are 232 °C and 328 °C respectively, whilst the solder melts at a lower temperature than either of these. (Lead-free solder tends to melt at about 220°C.) Thus the order of melting is: solder, tin and lead and the order of solidifying is the opposite.
Metal alloys are classified as solid solutions and are usually prepared by mixing together molten metals in an appropriate ratio.
If appropriate to the ability level, students should be asked to compare a conventional solid-liquid solution with that of an alloy.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet