Extend your learners’ understanding of energy changes that occur in changes of state, by exploring what happens when a liquid is supercooled
-

Download this
A ready-to-go practical lesson with classroom slides, integrated instructions, support and challenge student worksheets and teacher guidance, including full technical notes and answers to all questions.
Discover more resources from the Nuffield practical collection
In this experiment, learners melt sodium thiosulfate crystals, before cooling them to a state well below the melting point. The sodium thiosulfate will then exist in a metastable supercooled state. The supercooled liquid will freeze rapidly on the addition of a crystal of sodium thiosulfate or dust particles or on stirring. Learners add a crystal of the solid to seed the crystallisation process and observe temperature changes throughout.
The experiment is best carried out individually or in pairs and takes 20−30 minutes.
Learning objectives
- Make and record observations.
- Interpret a graph.
- Describe the energy changes that take place during a change of state.
Scaffolding
There are two versions of the student worksheet.
Use the level 1 support sheet (✪) to offer learners more scaffolding, including a ready-drawn results table and structured questions.
Use the level 2 challenge sheet (✪✪) to allow learners to make their own decisions about how to present their results and give more extended responses to questions.
For a safer alternative, which will also reduce experimental time, boil the hot water used to melt the sodium thiosulfate in a kettle, instead of asking students to set up a Bunsen burner, tripod and gauze.
As an alternative approach, set up a temperature sensor attached to a computer and project a continuous plot onto the screen as the temperature changes are observed. This will allow you to concentrate on LO2 and LO3 through the learner questions.
You can opt to collect some data using the data-logger while learners carry out their own experiment. This provides an opportunity to compare and evaluate the results.
Technician notes
Read our standard health and safety guidance and carry out a risk assessment before running any live practical.
Once the experiment is over, you can easily remove the thermometer by flushing with water (since sodium thiosulfate is water-soluble), or by re-melting the solid. Do not heat the boiling tube directly over a Bunsen flame as at higher temperatures the thiosulfate decomposes and may form toxic products.
Equipment
Apparatus
- Safety glasses
- Boiling tube (this must be very clean)
- Boiling tube holder
- Stirring thermometer, –10 °C to 110°C (A temperature sensor attached to a computer can be used in place of a thermometer. A continuous plot can then be drawn as the temperature changes occur)
- Beaker, 100 cm3
- Bunsen burner (or kettle to heat the water)
- Tripod and gauze
- Cotton wool, small tuft to fit boiling tube
Chemicals
- Sodium thiosulfate pentahydrate, about 20 g (Currently not classified as hazardous, see CLEAPPS hazcard HC095a. In Scotland, refer to SSERC for safety advice.)
Safety and hazards
Make sure that learners can use a Bunsen burner with confidence and remind them how to handle hot liquids in beakers, as in CLEAPSS student safety sheets SSS092 and SSS095.
Allow enough time for the equipment to cool down before asking learners to put away their equipment. In case of burns, follow the instructions on the CLEAPSS emergency cards E2a and cool the burn by immediately irrigating with gently running water for at least 20 minutes and until pain is relieved and heat is no longer felt.
Method
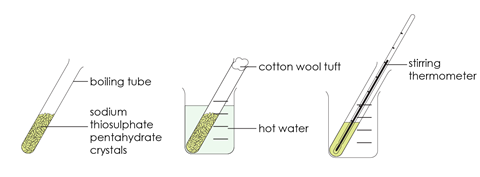
- Half-fill a very clean boiling tube with crystals of sodium thiosulfate pentahydrate.
- Put a tuft of cotton wool in the end of the boiling tube and place the boiling tube in a beaker of hot water (about 50°C) to melt the crystals.
- When all the crystals have melted, remove the cotton wool, put a thermometer in the melted sodium thiosulfate and record the temperature. If the liquid starts to crystallise on inserting the thermometer, reheat in water to melt all the solid.
- Stand the boiling tube in an empty beaker and leave to cool where it won’t be disturbed.
- Observe the temperature at various intervals until the value is in the region of 30−40°C. No crystallisation should have occurred.
- Add a fresh crystal of sodium thiosulfate pentahydrate, observe the rapid crystallisation which occurs and continue to monitor the temperature at regular intervals. Record your result in the table below.
- Continue to record your results until the temperature has fallen to about 25−30°C.
Teaching notes
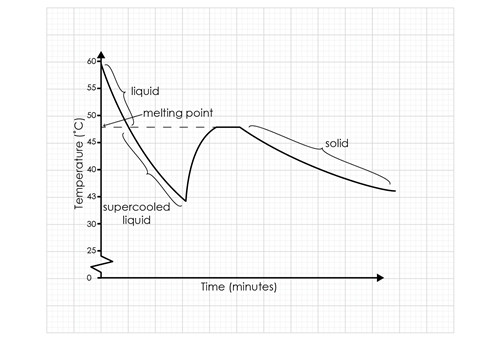
The temperature changes occurring show a steady fall as the liquid cools. When a crystal is added to the supercooled liquid, the temperature rapidly rises as solidification takes place, confirming this process is exothermic. The solid then cools to room temperature.
All solids exhibit supercooling to a greater or lesser extent, but sodium thiosulfate is particularly prone to exhibiting this metastable condition. It is possible to cool the liquid to a value well below room temperature, but to achieve this involves waiting for more time to elapse, lengthening the experiment considerably.
Carry out an impressive teacher demonstration using cold running water to cool the liquid rapidly to about 5−10°C before adding a crystal to seed the crystallisation process. The boiling tube will warm up rapidly.
Some vocabulary in this resource, such as metastable, might be unfamiliar to learners and require explanation.
Answers
Level 2 sheet
- (a) Freezing – a substance changes from a liquid to a solid. (b) Supercooled liquid – when a substance is in the liquid state below its freezing point. (c) Exothermic – when energy is transferred from the substances to the surroundings (usually in the form of heat). (d) Crystallisation – the process used to produce solid crystals from a concentrated solution.
- The graph should look similar to the sketch graph below. There should be suitable scales and points plotted correctly.
- See annotations on sketch graph below.
- The temperature increased/went up steeply. As the crystals were formed, the temperature increased rapidly. Heat energy was transferred/released to the surroundings, indicating an exothermic process had taken place.
- The answer should include the following points: In the liquid state, particles have enough kinetic energy to move freely. In the solid state, particles only have enough kinetic energy to vibrate around a fixed point. When a substance changes from the liquid state to the solid state, the excess energy is transferred from the supercooled liquid to the surroundings as heat energy. The temperature increases. The process is exothermic.
Level 1 sheet
- Freezing: When a substance changes from a liquid to a solid. Crystallisation: The process used to produce solid crystals from a concentrated solution. Supercooled liquid: When a substance is in the liquid state below its freezing point. Exothermic: When energy is transferred from a substance to the surroundings, usually in the form of heat.
-
(a) Crystallisation taking place − D
(b) Liquid sodium thiosulfate − A
(c) Supercooled liquid sodium thiosulfate − B
(d) Solid sodium thiosulfate − E
(e) sodium thiosulfate crystal added − C
-
The graph increased/went up steeply.
-
The temperature increased as the crystals were formed.
Downloads
Supercooling student support sheet
Handout | PDF, Size 0.3 mbSupercooling student challenge sheet
Handout | PDF, Size 0.29 mbSupercooling presentation slides
Handout | PDF, Size 0.84 mbSupercooling teacher notes
PDF, Size 0.35 mbSupercooling student support sheet
Editable handout | Word, Size 0.68 mbSupercooling student challenge sheet
Editable handout | Word, Size 0.64 mbSupercooling presentation slides
Editable handout | PowerPoint, Size 3.66 mbSupercooling teacher notes
Editable handout | Word, Size 0.65 mb
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. The supporting resources were updated in 2025 by Dorothy Warren.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry
































No comments yet