The temperature profile of this exothermic reaction can be used to identify the end point
In this video from Malmesbury Education Mr Mitchell shows you how to measure the temperature change of an exothermic reaction.
The video explains that at the end of the reaction you should start to see the temperature level off or decrease. Below are some typical results for this practical from the AQA Required Practical Handbook. Students should be able to calculate the mean maximum temperature to complete the table.
| Total volume of Sodium Hydroxide added in cm3 | Maximum temperature in °C | ||
|---|---|---|---|
| First trial | Second trial | Mean | |
| 0 | 20.0 | 21.0 | |
| 5 | 24.0 | 24.6 | |
| 10 | 26.8 | 27.6 | |
| 15 | 28.6 | 29.6 | |
| 20 | 30.8 | 31.3 | |
| 25 | 31.8 | 32.8 | |
| 30 | 32.0 | 32.6 | |
| 35 | 31.6 | 31.8 | |
| 40 | 30.6 | 31.0 | |
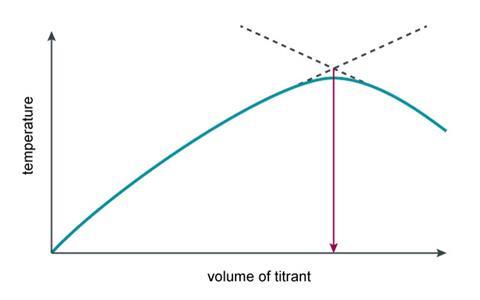
These results can be used to identify the volume of sodium hydroxide that was needed to neutralise the hydrochloric acid. Students should be guided towards drawing two lines of best fit which intersect to give the end point of the reaction and can be used to find the volume of sodium hydroxide.
Real-world contexts
- Learn how endothermic reactions could be used to cool houses with this science research news summary slide.
- With these activities learners can experience energy changes to make cool packs and ice cream.
- Meet Robert, a consumer products technician, who studies the behaviour of different materials in order to develop and improve products such as cold packs used to treat sports injuries.
- Instigate a discussion with learners, using this concept cartoon about birthday cake candles.
Also check out
- Monitoring reactions – this article provides an overview of related key concepts in the curriculum, with ideas, questions and links to more resources.
- Acids and bases: creating solutions – an article addressing common misconceptions and students’ difficulties surrounding this topic.
- A hot dinner from a can – challenge students to produce a design and create a proto-type of a self-heating can of food.
Additional information
We have collated these videos of key practical experiments to support remote teaching as part of our response to Covid-19. Teachers requested resources to help them deliver practical content without access to laboratories or equipment. We are developing further resources and welcome feedback to help us produce those you most need. Please email us or use the comment section below.
Real-world contexts added by Ian McDaid.
Practical videos | 14–16 years

Videos of core practical experiments for flipped learning, remote teaching or revision
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
 Currently
reading
Currently
reading
Temperature change (neutralisation)
- 14











































7 readers' comments