Create fabric dye by synthesising an azo dye and using it to change the colour of a piece of cotton
In this experiment, students prepare an azo dye and use it to dye a piece of cotton. The synthesis is unusual in that whereas most organic syntheses require ambient or elevated temperature, this synthesis requires low temperatures.
This practical should take 20 minutes.
Equipment
Apparatus
- Beakers, 10 cm3, x3
- Thermometer
- Tweezers
Chemicals
- Ice
- Aminobenzene (aniline)
- Hydrochloric acid (concentrated)
- Sodium nitrite
- 2-Naphthol (also called β-Naphthol; naphthalene-2-ol)
- Sodium hydroxide solution, 2 mol dm–3
- Ethanol
- Urea
Health, safety and technical notes
- Read our standard health and safety guidance.
- Students must wear suitable eye protection (splash resistant goggles to BS EN166 3).
- This experiment should be done in a fume cupboard.
- Aminobenzene (aniline) is TOXIC by all routes, a carcinogen and mutagen, a skin sensitiser, causes eye damage and is toxic to aquatic life .
- Hydrochloric acid, HCl(aq), is CORROSIVE and a respiratory irritant (see CLEAPSS HazCard HC047a).
- Sodium nitrite, NaNO2, is an OXIDISER, toxic if swallowed and hazardous to aquatic life (see CLEAPSS HazCard HC093).
- 2-Naphthol is HARMFUL if swallowed or in contact with skin and toxic to aquatic life (see CLEAPSS HazCard HC070b).
- Sodium hydroxide solution, NaOH(aq), 2 mol dm–3 is CORROSIVE (see CLEAPSS HazCard HC091a).
- Ethanol is highly FLAMMABLE (see CLEAPSS HazCard HC040a).
- Urea is of low hazard (see CLEAPSS HazCard HC035a).
Procedure
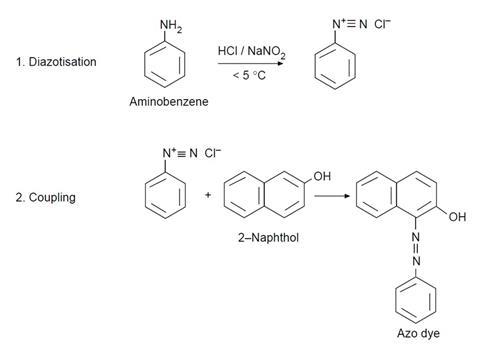
- Put eight drops of aminobenzene in a 10 cm3 beaker and add 30 drops of deionised water followed by 15 drops of concentrated hydrochloric acid. Swirl the beaker and then put it in an ice bath.
- Weigh 0.15 g of sodium nitrite into another beaker and add 1 cm3 of deionised water. Cool the beaker in the ice bath. Add one spatula of urea (this prevents side reactions occurring).
- Mix the contents of the two beakers together and keep in the ice bath.
- Weigh 0.45 g of 2-naphthol into another beaker and add 3 cm3 of sodium hydroxide solution. Swirl to dissolve.
- Take a piece of cotton cloth 2 × 2 cm2 and, using tweezers, dip it into the 2-naphthol solution. Allow the solution to completely soak the cotton.
- Dip the cloth completely into the diazonium salt solution. A red dye forms in the fibres, dyeing the cloth.
- Take the cloth out, wash it under the tap and leave to dry.
Observations
The orange-red azo dye forms in the fibres of the cotton, dyeing the cloth. The melting point of 1-phenylazo-2-naphthol is 133 °C.
The urea decomposes excess HNO2 formed and prevents many side reactions from occurring. A better ‘red’ dye is usually produced.
Downloads
The microscale synthesis of azo dyes - student sheet
Experiment | PDF, Size 0.16 mbThe microscale synthesis of azo dyes - student sheet
Editable handout | Word, Size 95.05 kbThe microscale synthesis of azo dyes – teacher notes
Experiment | PDF, Size 0.12 mbThe microscale synthesis of azo dyes – teacher notes
Editable handout | Word, Size 53.24 kb
References
S. W. Breuer, Microscale practical organic chemistry. Lancaster: Lancaster University, 1991.
Additional information
This resource is part of our Microscale chemistry collection, which brings together smaller-scale experiments to engage your students and explore key chemical ideas. The resources originally appeared in the book Microscale chemistry: experiments in miniature, published by the Royal Society of Chemistry in 1998.
© Royal Society of Chemistry
Health and safety checked, 2018


















No comments yet