All Structure and bonding articles – Page 10
-
![Img 5543 colour corrected 300x225 tb[1]](https://d1ymz67w5raq8g.cloudfront.net/Pictures/100x67/5/9/1/113591_img_5543_colourcorrected_300x225_tb1.jpg) Opinion
OpinionThe education of Harry Kroto
Kristy Turner reflects on Sir Harry Kroto's relationship with education
-
 Feature
FeatureThe origin of life
How did molecules turn into living organisms? Paul MacLellan investigates
-
 Feature
FeatureThe bonds that bind
Mike Sutton plots the journey of the scientists who solved the riddle of chemical bonding
-
 News
NewsBoron nitride composites stronger than their carbon cousins
Binding strengths of polymer–boron nitride nanotubes are higher than those reported for carbon nanotubes
-
 Resource
ResourceOrganising your understanding
Three activities that progressively stretch learners’ understanding of these key topics using Venn-like diagrams to organise information
-
 Demonstration
DemonstrationThe density of ice
Demonstrate to students what happens as ice cubes floating on oil start to melt and the density of the water changes. Includes kit list and safety instructions.
-
 Resource
ResourceConducting polymers and shape memory polymers │16–18 years
Worksheets on conducting polymers and shape changing polymers
-
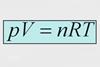 Resource
ResourceChemistry Vignettes: Physical Chemistry
A series of short screencasts to introduce aspects of physical chemistry.
-
 Resource
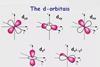
ResourceChemistry Vignettes: Transition Metal Fundamentals
A series of short screencast videos to introduce transition metal chemistry
-
 Resource
ResourceChemistry Vignettes: Transition Metal Chemistry
A series of short screencast videos to introduce transition metal chemistry.
-
 Resource
ResourceChemistry Vignettes: NMR Theory
A series of short screencast videos to introduce NMR spectroscopy Video: NMR: Atoms and Molecules in a Magnetic Field Video: NMR: Nuclei in a Magnetic Field Video: NMR: Fine structure
-
 Resource
ResourceChemistry Vignettes: IR Spectroscopy Theory
A series of short screencast videos to introduce IR spectroscopy
-
 Resource
ResourceChemistry Vignettes: Molecular Orbital Theory
A series of short screencast videos to introduce acids and bases
-
 Resource
ResourceChemistry Vignettes: Bonding Theory and VSEPR
A series of short screencast videos to introduce bonding theory.
-
 Resource
ResourceChemistry Vignettes: Advanced Transition Metal Chemistry
A series of short screencast videos to introduce transition metal chemistry
-
 Resource
ResourceGraphene: future applications
Watch a video explaining the types of carbon, making graphite, the properties and uses of graphite
-
 Feature
FeatureSeeing is believing
Elinor Hughes discovers the technique that has imaged molecules directly for the first time
-
![Shutterstock 195864272 300tb[1]](https://d1ymz67w5raq8g.cloudfront.net/Pictures/100x67/6/3/8/113638_shutterstock_195864272_300tb1.jpg) Opinion
OpinionFive ideas in chemical education that must die: The atomic orbital conundrum
Are 4s atomic orbitals preferentially occupied and ionised?
-
 Demonstration
DemonstrationAllotropes of sulfur
Use this practical to explore the changes in the colour and consistency of sulfur as you heat it, melt it and eventually boil it. Includes kit list and safety instructions.
-
 Class experiment
Class experimentThe structure and properties of chocolate
Investigate how melting chocolate changes its structure and affects properties like taste, texture and melting point. Includes kit list and safety instructions.











