All Analysis articles – Page 7
-
 Demonstration
DemonstrationIdentifying the products of combustion
Illustrate the presence of water and carbon dioxide in the products of hydrocarbon combustion in this demonstration. Includes kit list and safety instructions.
-
 Class experiment
Class experimentChromatography of sweets | 11–14 years
Try this class practical to carry out chromatography using dye from different coloured M&M’s®. Includes kit list and safety instructions.
-
 Demonstration
DemonstrationSilver and lead halides
Try this practical or demonstration to produce silver and lead halides in a series of precipitation reactions. Includes kit list and safety instructions.
-
 Resource
ResourceProblem based practical activities
Discover how chemistry can relate to real world problems, so students can put their science knowledge into context.
-

-
 Soundbite
SoundbiteDual personality of light caught on camera
Is it a wave or is it a particle? We might know the answer, (Spoiler alert: it’s both!) but it is reassuring nonetheless to see the pictures that prove it, says Nina Notman
-
 Feature
FeatureThe impossible water sensor
Hundreds of different chemicals can ruin our water, so measuring their levels is vital. Josh Howgego investigates whether building sensors that can do the job cheaply and remotely will ever be possible
-
 Resource
ResourcePlastics conservation - Barbie and friends
This resource provides the essential knowledge needed to keep plastic pieces in the best possible condition so that they can continue to be enjoyed for many years.
-
 Resource
ResourceWhich sodium salt is which?
How does sodium interact with other chemicals, and how can you identify them? Includes kit list and safety instructions.
-
 Resource
ResourceWhich gas is which?
Five mystery gases, and a problem to solve. Students must identify each gas, and do so quickly. Includes kit list and safety instructions.
-
 Resource
ResourceVintage titrations: sulfur dioxide concentrations in wine
Explore redox titrations using iodine, and discover the sulfur dioxide concentrations in wine. Includes kit list and safety instructions.
-
 Resource
ResourceVintage titrations: tannin in wine
Explore Redox titrations using potassium manganate(VII), with this tannin experiment. Includes kit list and safety instructions.
-
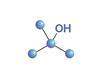 Resource
ResourceThree isomeric alcohols
Learn about the reactions of primary, secondary and tertiary alcohols, using laboratory tests. Includes kit list and safety instructions.
-
 Resource
ResourceOnly dust - is there a sign of life?
Discover the link between organic life, and dust, designing an experiment of your own from junk materials. Includes kit list and safety instructions.
-
 Feature
FeatureIdentifying extraterrestrial materials
Meteorites can be bought cheaply online and offer an excellent laboratory teaching tool, explain Luis Lahuerta Zamora, Salvador Lahuerta Zamora and Ana Mellado Romero
-
 Resource
ResourceRi Christmas Lectures® 2012: Group 1 Flame Tests
A teaching resource on the group 1 flame tests, supported by video clips based around the Royal Institution 2012 Christmas Lectures® Video: Group 1 flame tests Video: Lithium flame test Video: Sodium Flame Test Video: Potassium ...
-
 Feature
FeatureGood chemistry
There are all sorts of ways chemists can use their skills to aid global development, writes Josh Howgego
-
 Feature
FeatureThe art detectives
Emma Stoye finds out how spectroscopic techniques allow scientists to look over the shoulders of old masters
-
-
 The Mole
The MoleThe monks’ tales
Kathryn Roberts looks at how modern spectroscopy lets us discover the secrets of 1500-year-old manuscripts without leaving the library











