Try this microscale class practical to analyse aspirin tablets and find out how much salicylic acid is present
In this experiment, students measure the amount of free 2-hydroxybenzoic acid (salicylic acid) in 2-ethanoyloxybenzenecarboxylic acid (aspirin) tablets.
2-hydroxybenzoic acid (salicylic acid), being a substituted phenol, reacts with Fe3+ ions to produce a purple colour. The colour is matched against that produced by a set of standard solutions of 2-hydroxybenzoic acid (salicylic acid) in a well-plate.
The practical should take approximately 20 minutes.
Equipment
Apparatus
- Eye protection
- 24-well plate
- Beaker, 100 cm3
- Cotton wool
- Plastic pipette (standard form, eg Aldrich ref: Z13, 500-3)
- Plastic pipettes (fine tip, eg Aldrich ref: Z13, 503-8), x2
- Sheet for microscale filtration technique (see note 4 below)
Chemicals
Note
Solutions should be contained in plastic pipettes (fine tip). See the accompanying guidance on apparatus and techniques for microscale chemistry, which includes instructions for preparing a variety of solutions.
- Various 2-ethanoyloxybenzenecarboxylic acid (aspirin) tablets
- Iron(III) nitrate solution, 0.1 mol dm–3
- 2-hydroxybenzoic acid (salicylic acid) (working) solution
- Deionised water
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Advise students not to ingest the aspirin tablets.
- See our guidance on apparatus and techniques for microscale chemistry for microscale filtration instructions.
- Iron(III) nitrate, Fe(NO3)3.9H2O(aq), 0.1 mol dm–3 is of low hazard. See CLEAPSS Hazcard HC055C and CLEAPSS Recipe Book RB052.
- 2-hydroxybenzoic acid (salicylic acid), used in preparation of the stock solution, DANGER this substance is harmful if swallowed, is suspected of damaging the unborn child, is harmful to aquatic life with long-lasting effects and may cause an allergic skin reaction, see CLEAPSS Hazcard HC052.
- 2-hydroxybenzoic acid (salicylic acid) solution (0.1% w/v) is of low hazard. See CLEAPSS Hazcard HC052.
- Ethanol/water mixture (1:1) is FLAMMABLE. See CLEAPSS Hazcard HC040A and CLEAPSS Recipe Book RB002.
Preparing a stock salicylic acid solution (0.1% w/v)
Dissolve 0.100 g of 2-hydroxybenzoic acid (salicylic acid) in approximately 20 cm3 of a 1:1 mixture of ethanol and deionised water in a 100 cm3 beaker. Make up to 100 cm3 in a volumetric flask.
Preparing a working salicylic acid solution
To produce a working solution (0.0025 g 2-hydroxybenzoic acid (salicylic acid) / 25 cm3), dilute 2.5 cm3 of the stock solution to 25 cm3 in a volumetric flask with a 1:1 ethanol–water mixture.
Procedure
Part 1: preparation of standard solutions
In this part of the experiment, students prepare a set of standard solutions with different colour intensities from the standard 2-hydroxybenzoic acid (salicylic acid) solution. They use these to match the intensity of the colour produced from the 2-ethanoyloxybenzenecarboxylic acid (aspirin) solution in part 2, and so find out how much 2-hydroxybenzoic acid (salicyclic acid) there is in their 2-ethanoyloxybenzenecarboxylic acid (aspirin) tablet.
Taking their 24-well plate, they add drops of solutions as indicated in the table on their worksheet.
Part 2: analysis of aspirin tablets
- Record the mass of a 2-ethanoyloxybenzenecarboxylic acid (aspirin) tablet and place it in a 100 cm3 beaker.
- Add 10 cm3 of the 50% ethanol–water mixture (from a measuring cylinder) and swirl the mixture. The tablet will begin to disintegrate.
- Using the microscale filtration method, filter the mixture into a 25 cm3 volumetric flask. Wash the beaker with a small quantity of the ethanol–water mixture and add to the flask. Make up to the mark, stopper and mix.
- Add 50 drops of this 2-ethanoyloxybenzenecarboxylic acid (aspirin) solution to well B3 followed by five drops of the iron(III) nitrate solution.
- Match the colour to that of one of the standard solutions.
Calculations
Calculate the percentage of 2-hydroxybenzoic acid (salicylic acid) in the 2-ethanoyloxybenzenecarboxylic acid (aspirin) tablet as follows:
- Identify the standard well that matches the colour intensity of the 2-ethanoyloxybenzenecarboxylic acid (aspirin) sample well.
- The mass of 2-hydroxybenzoic acid (salicylic acid) (in 25 cm3) in the solution from this standard well is therefore the same as the mass of 2-hydroxybenzoic acid (salicylic acid) in the 25 cm3 of solution of your 2-ethanoyloxybenzenecarboxylic acid (aspirin) tablet solution.
- Divide this mass (mg) by the mass of your 2-ethanoyloxybenzenecarboxylic acid (aspirin) tablet (mg) and multiply this value by 100 to give a percentage by mass.
Question for students
By considering the equation for the formation of 2-hydroxybenzoic acid (salicylic acid) from 2-ethanoyloxybenzenecarboxylic acid (aspirin), are there any differences in how much 2-hydroxybenzoic acid (salicylic acid) is present in both old and new bottles of 2-ethanoyloxybenzenecarboxylic acid (aspirin) tablets?
Teaching notes and expected observations
The set of standard solutions should give a range of intensities of a bluish colour. Students should be careful to add the correct number of drops as indicated. The experiment works best with old tablets containing some free 2-hydroxybenzoic acid (salicylic acid). New tablets with minimal free acid do not give any blue coloration but merely the colour of iron(III) in solution (yellow) so they do not fit into the range of standards.
The equation by which 2-hydroxybenzoic acid (salicylic acid) is formed is:
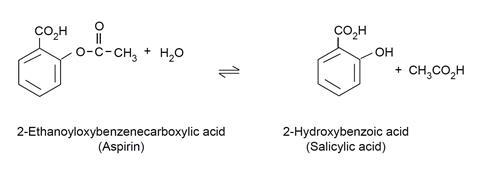
This experiment gives students an opportunity to consider the practical effect of equilibrium. Old 2-ethanoyloxybenzenecarboxylic acid (aspirin) tablets, which may have become damp with time, will contain more free 2-hydroxybenzoic acid (salicylic acid) because the presence of water causes the position of equilibrium to be shifted to the right in the above equation.
Downloads
Analysis of aspirin on a microscale - student sheet
Editable handout | Word, Size 0.11 mbAnalysis of aspirin on a microscale - student sheet
Handout | PDF, Size 0.19 mbAnalysis of aspirin on a microscale - teacher notes
Editable handout | Word, Size 0.12 mbAnalysis of aspirin on a microscale - teacher notes
Handout | PDF, Size 0.22 mb
Additional information
This resource is part of our Microscale chemistry collection, which brings together smaller-scale experiments to engage your students and explore key chemical ideas. The resources originally appeared in the book Microscale chemistry: experiments in miniature, published by the Royal Society of Chemistry in 1998.
© Royal Society of Chemistry
Health and safety checked, 2018


















1 Reader's comment