Try this worked example of a Chemistry Olympiad question on the chemistry behind matches, including enthalpy change and NMR spectroscopy
In this question from the 2009 UK Chemistry Olympiad round one paper, students learn about the reaction that takes place when a match is struck. They write equations for the combustion of phosphorus sesquisulfide, as well as the decomposition of potassium chlorate(V), before turning to the reaction between these two substances and calculating a mass ratio and enthalpy change. They then compare the structures of various phosphorus sulfides as determined by NMR spectroscopy.
The accompanying video walkthrough is designed to help students if they get stuck or want to check their working.
This question is of moderate difficulty, and covers the following chemistry topics:
- Balancing equations
- Mole calculations
- Enthalpy calculations
- NMR spectroscopy
What’s on this page?
- How to use this resource
- Question
- Answers
- Walkthrough (including video and transcript)
How to use this resource
Find out more about how to use Chemistry Olympiad worked answers.
Note
This question first appeared as question 3 in the 2009 UK Chemistry Olympiad round one paper. For marking, including notes on allowances, see question 3 in the 2009 examiners’ mark scheme.
Question
The heads of strike-anywhere matches contain a mixture of phosphorus sesquisulfide P4S3 and potassium chlorate(V) KClO3. When the match is struck across a rough surface the heat of friction is sufficient to ignite the phosphorus sesquisulfide; the potassium chlorate(V) decomposes to provide the oxygen needed for combustion.
Part a
- Write an equation to show the combustion of phosphorus sesquisulfide into phosphorus(V) oxide and sulfur dioxide.
- Write an equation to show the decomposition of potassium chlorate(V) into potassium chloride and oxygen.
- Hence or otherwise write a single equation to show the reaction that takes place between these two substances when a match-head ignites.
- Calculate the mass ratio in which phosphorus sesquisulfide and potassium chlorate(V) should be combined on the match-head.
- Given the standard molar enthalpy changes of formation in the table below, calculate the standard enthalpy change for the reaction in part (a)(3).
| KCl(s) | KClO3(s) | SO2(g) | P4S3(s) | P4O10(s) | |
|---|---|---|---|---|---|
| ΔfHꝋ kJ mol–1 | –436.7 | –397.7 | –296.8 | –154.0 | –2948 |
Part b
Phosphorus sulfides can be made by heating white phosphorus with sulfur. When this reaction is carried out at low temperature a range of products from P4S3 to P4S10 are produced. 31P NMR spectroscopy has been used to determine the structures of many of the phosphorus sulfides, such as those below.
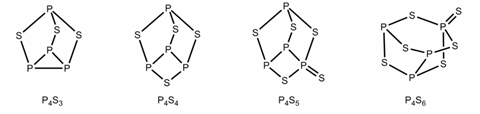
In 31P NMR spectroscopy the number of peaks seen corresponds to the number of different P environments, for example P4S3 has two different P environments so shows two peaks in the 31P NMR spectrum.
Using the structures above, predict how many peaks would be seen in the 31P NMR spectrum of:
- P4S4
- P4S5
- P4S6
Part c
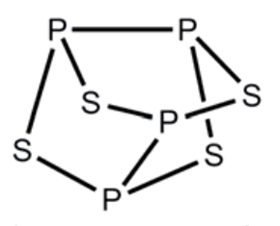
P4S4 has, in fact, been shown to exist in two different isomeric forms. You are given the structure of one isomer above. The second isomer shows only one peak in the 31P NMR spectrum.
Suggest a structure for the second isomer of P4S4.
Answers
Part a
- P4S3 + 8O2→ P4O10 + 3SO2
- 2KClO3→ 2KCl + 3O2
- 3P4S3 + 16KClO3→ 3P4O10 + 9SO2 + 16KCl
- P4S3/ KClO3 = 660 / 1961 = 1 / 2.97
- Δr HØ = ( (3 x –2948) + (9 x –296.8) + (16 x –436.7 ) ) – ( (3 x –154.0) + (16 x –397.7 ) ) = –11700 kJ mol–1
Part b
- 3 peaks
- 4 peaks
- 3 peaks
Part c
As below, or any other reasonable structure, that fits with the data and with elements in correct valencies.
Walkthrough
Transcript
Download this
Introduction
This question is all about the chemistry of matches.
Part a
Part (a) of the question looks at the reaction that occurs on the head of the match when the match is struck. The match head contains a mixture of two chemicals, phosphorus sesquisulfide P4S3 and potassium chlorate(v), KClO3. When the match is struck, the heat of friction is sufficient to ignite the phosphorus sesquisulfide. The potassium chlorate(V) decomposes to provide the oxygen needed for the combustion.
In part (1), you are asked to write an equation to show the combustion of phosphorus sesquisulfide into phosphorus(V) oxide and sulfur dioxide. The formula for phosphorus sesquisulfide is given in the question as P4S3. This undergoes combustion, which requires oxygen, to form phosphorus(V) oxide and sulfur dioxide. You may not be aware of the formula of phosphorus(V) oxide but you can work it out using the oxidation states. If the phosphorus has an oxidation state of +5 and you know that oxygen usually has an oxidation state of –2 then since compounds are electrically neutral and hence the sum of all the oxidation states in a compound = 0, you can work out that the phosphorus and oxygen must combine in a ratio of 2 phosphorus to 5 oxygen. You will get the marks if you balance the equation using P2O5 as the formula of phosphorus(V) oxide. However, phosphorus pentoxide in fact exists as a dimer, P4O10. You can see this if you look further down the question to the table.
Having correctly worked out the formulae of the products of the reaction all that remains now is to balance the equation, balancing each of the elements in turn. There are four atoms of phosphorus on both sides of the equation and so the phosphorus is balanced. Three atoms of sulfur go into the equation and so three molecules of SO2 must be produced. Finally, balancing the oxygen atoms, ten plus six oxygen atoms are produced and so eight molecules of O2 must be added to the left.
In part (2) of the question you are asked to look at the other half of the reaction which occurs on the match head, which is the decomposition of potassium chlorate(V) into potassium chloride and oxygen. Since you are either given or know the formulae of each of the reactants and products in this case this is much simpler. As the reaction is a decomposition, the potassium chlorate(V) simply breaks down to produce potassium chloride and oxygen gas. Balancing the equation, if one molecule of potassium chlorate(V) undergoes decomposition, then one and a half molecules of oxygen are produced. To remove the fractions from the equation, multiply the complete equation by two, to produce a final equation of 2KClO3 decomposes to form 2KCl + 3O2.
In part (3) of the question you are now asked to combine these two equations to produce a single equation to show the reaction that takes place between these two substances when a match-head ignites. In order to do this, it is necessary to ensure that the number of molecules of oxygen produced by the potassium chlorate(V) matches the number of molecules of oxygen needed for the combustion of the phosphorus sesquisulfide. Since a common multiple of 3 and 8 is 24, you can multiply the equation produced in part (1) by 3 and the equation produced in part (2) by 8 and combine the two equations. This leaves 24 molecules of oxygen on the left, and 24 molecules of oxygen on the right which can be cancelled out to give a final equation for the reaction that takes place on the match head as:
3P4S3 + 16KClO3 → 3P4O10 + 9SO2 + 16KCl
Part (4) of the question asks you to use the equation you produced in part (3) to calculate the mass ratio in which phosphorus sesquisulfide and potassium chlorate(V) should be combined on the match head. From the equation, you know that 3 moles of P4S3 react with 16 moles of KClO3. In order to convert this mole ratio into a mass ratio you need to use the equation mass = moles multiplied by molar mass. Using the relative atomic masses given in the periodic table at the start of the question paper, it is possible to calculate the molar mass of phosphorus sesquisulfide as 220.06 g mol–1 and the molar mass of potassium chlorate(V) as 122.552 g mol–1. Applying these values and the mole ratios to the equation above, the mass of phosphorus sesquisulfide needed is seen to 660.18 g and the mass of potassium chlorate(V) needed is seen to be 1960.832 g. Hence the two chemicals must be combined in a ratio of 1:2.97.
In the final part, part (5) of part(a), you are given the standard molar enthalpy changes of formation for each of the reactants and products involved in the reaction on the match head, and asked to calculate the standard enthalpy change for this reaction. The standard enthalpy change of formation is the enthalpy change when one mole of a compound is formed from its constituent elements under standard conditions. Thus the standard molar enthalpy changes of formation can be represented by an arrow going from the elements to the compound whose enthalpy change of formation you are given. A value can be added to that arrow to represent the enthalpy change for the formation of the number of moles of compound in question. Hess’s law states that the enthalpy change for a chemical reaction is the same whatever route is taken from reactants to products. Therefore, Hess’s law can be used to calculate the enthalpy change for this reaction.
For the reactants, you need to go from the compound to the elements from which it is formed. This is the reverse of the enthalpy of formation and hence you these values must be subtracted. For the products, you need to move from the elements to the compounds and hence these values are added. Therefore, our calculation for the enthalpy change of the reaction on the match head becomes minus 3 × –154.0 minus 16 × –397.7 + 3 × –2,948 plus 9 × –296.8 plus 16 × –436.7 kJ mol–1. With calculations such as this it is very easy to make a silly mistake when placing the numbers into the calculator. It is good practice therefore to write out the answer for each individual calculation and then complete the final calculation in a separate step. If you do this you find that the enthalpy change for the reaction on the match head is equal to +462.0 + 6,363.2 – 8,844 – 2,671.2 – 6,987.2 which equals –11,677.2 kJ mol–1 or –11,700 kJ mol–1 to 3 significant figures. You notice here that this is a very large negative enthalpy change showing us that the reaction is highly exothermic as you would expect.
Part b
The question now moves on to look at the phosphorus sulfides in more detail. You are told that phosphorus sulfides can be made by heating white phosphorus with sulfur. When this reaction is carried out at low temperature, a range of products from P4S3 to P4S10 are made. 31P NMR has been used to determine the structures of many of the sulphides and diagrams of the structures of some phosphorus sulfides are shown.
In part (b) of the question you are asked to predict how many peaks would be seen in the 31P NMR spectrum of three of these phosphorus sulfides. Although you won’t have met 31P NMR in your studies, it is very similar to 13C or 1H NMR and you are in fact told that in 31P NMR the number of peaks seen corresponds to the number of different phosphorus environments. To answer this question, we need to look at each of the three sulfides in turn, you need to look for any symmetry in the molecules which would create phosphorus atoms in equivalent environments.
In P4S4 you can see that there is a plane of symmetry through the middle of the molecule. Therefore the two phosphorus atoms highlighted are in equivalent chemical environments and so you would expect to see 3 peaks in the 31P NMR spectrum. In P4S5 the P=S removes all symmetry from the molecule and so in this case all 4 phosphorus atoms are in non-equivalent environments and so you would expect to see 4 peaks in the 31P NMR spectrum. Finally in P4S6 the two P atoms highlighted are in identical chemical environments owing to the plane of symmetry which cuts through the middle of the P-P bond. Therefore you would expect to see 3 peaks in the 31P NMR spectrum as a result of the 3 different P environments highlighted.
Part c
Finally you are told that P4S4 can exist in two different isomeric forms, only one of which is shown. The second isomer shows only one peak in the 31P NMR spectrum. Part (c) of the question asks you to suggest a structure for this second isomer. When drawing the structure it is important to ensure that each element has the correct number of bonds. Phosphorus is in group 5 and so needs to form three covalent bonds to fill its outer shell. Sulfur is in group 6 and so needs to form only two covalent bonds.
Since the 31P NMR spectrum of the second isomer shows only a single peak, the molecule must have a high degree of symmetry. From maths perhaps or studies on the shapes of molecules you may realise that the most symmetric way to arrange four atoms is at the four corners of a tetrahedron. We know we can’t join all the phosphorus atoms with a sulfur bridge as this would require the formula of the molecule to be P4S6, but if we bond the phosphorus atoms together in pairs and then add sulfur bridges to each of the remaining edges we create a highly symmetrical structure with the correct formula and the correct valency for each element in which all four phosphorus atoms are in identical chemical environments.
Downloads
Enthalpy and the chemistry of matches - video transcript
Editable handout | Word, Size 0.98 mbEnthalpy and the chemistry of matches - video transcript
Handout | PDF, Size 0.62 mb
Chemistry Olympiad worked answers
- 1
- 2
- 3
- 4
- 5
 Currently reading
Currently readingEnthalpy and the chemistry of matches
- 6
- 7
- 8

























No comments yet