Quiz your students on their equilibria knowledge
Topics covered in this starter for ten activity are: dynamic equilibria, Le Châtelier’s principle and equilibria and industry.
Example questions
This question is about the equilibrium established between hydrogen, iodine and hydrogen iodide;
H2 + I2 ⇌ 2 HI
1.(a) If the system is at equilibrium, indicate whether the statements below are True or False; (6 marks)
The rate of the forward and backward reaction must be the same
The concentration of the reactants and products is the same
The equilibrium must have been established by reacting hydrogen with iodine
The system must be sealed
Iodine is purple in colour. Hydrogen and hydrogen iodide are colourless. Therefore as the iodine is used up, the colour of the system will gradually fade.
The pressure of the system will remain constant
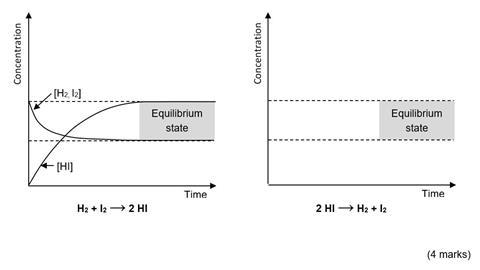
(b) The diagram on the left below shows how the concentration of reactants and products changes when the equilibrium is established from an equimolar mixture of hydrogen and iodine. Draw an equivalent diagram on the axes on the right to show how the concentration of reactants and products would change if the equilibrium was established from pure hydrogen iodide under the same conditions.
Notes
A full version of this question and answer sheet is available from the ‘Downloads’ section below. An editable version is also available.
Downloads
Equilibria
PDF, Size 0.27 mbEquilibria
Word, Size 0.14 mb
Starters for 10: Advanced level 1 (16–18)
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
 Currently reading
Currently readingEquilibria
- 10
- 11
- 12




























No comments yet