Solidify your students’ understanding of the structure and properties of metals and alloys
Metallic bonding is a type of strong chemical bond that occurs in pure metals and alloys.
-

-

Scaffolded worksheets
Use the accompanying activity to assess learners’ understanding of structure and bonding in metals and alloys with true and false, identify-the-error and long-answer questions. The scaffolded student worksheet includes prompts and a structure strip to offer more support for learners
View and download more infographics

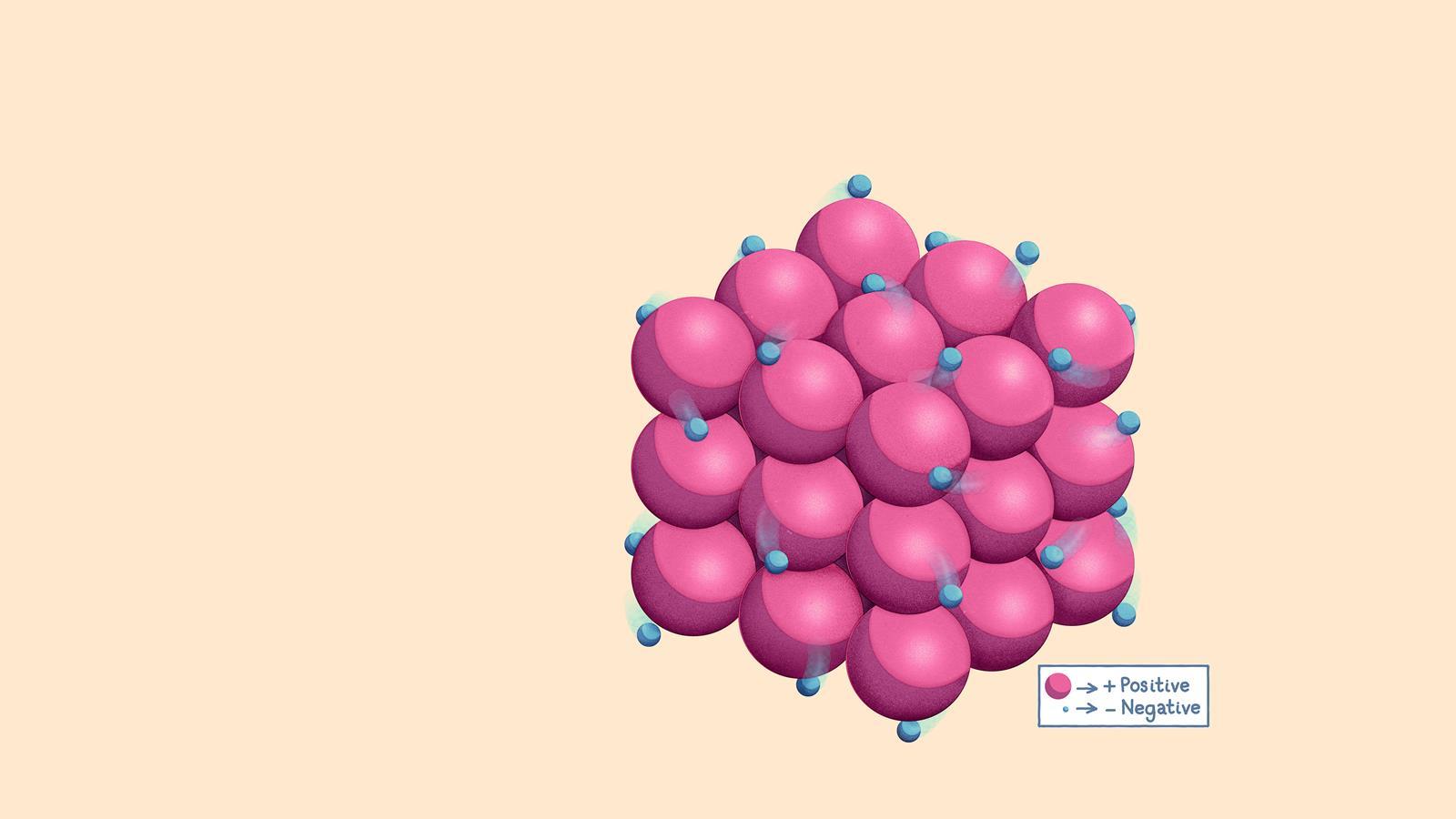
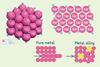
Metals are giant three-dimensional structures where layers of positive metal ions are surrounded by a sea of delocalised outer-shell electrons
The metal ions in the three-dimensional lattice structure are closely packed. This means that metals have a high density
Did you know …?
The chemical formula of a metal is just the symbol for the element as metallic lattices do not contain a fixed number of atoms – eg sodium is represented as Na.
Structure and properties
A large amount of energy is required to overcome metallic bonds, so metals and alloys have high melting and boiling points.
Metals are good electrical conductors because the delocalised electrons are free to move through the structure and carry electrical charge.
The movement of electrons allows thermal energy to pass efficiently through metals, which means they are good thermal conductors too.
Did you know …?
The melting and boiling points of metals are related to the number of outer shell electrons. The greater the charge of the metal ion, the greater the number of delocalised electrons and the stronger the bonds.
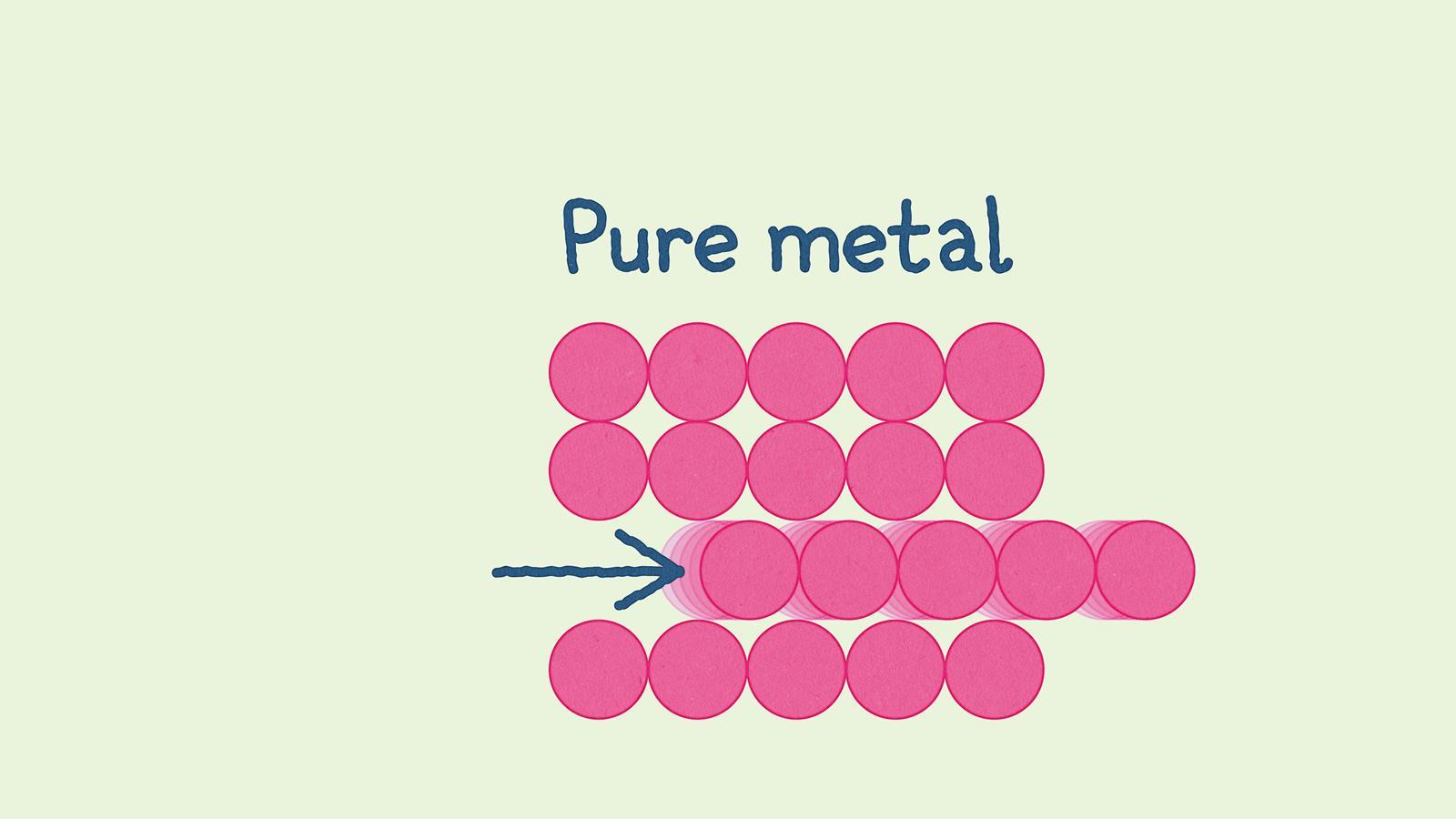
Pure metals only contain one type of metal atom, so the atoms are arranged in layers which can slide over one another. This means they are malleable – can be hammered or pressed into shape without breaking or cracking – and ductile, so they can be drawn into wires
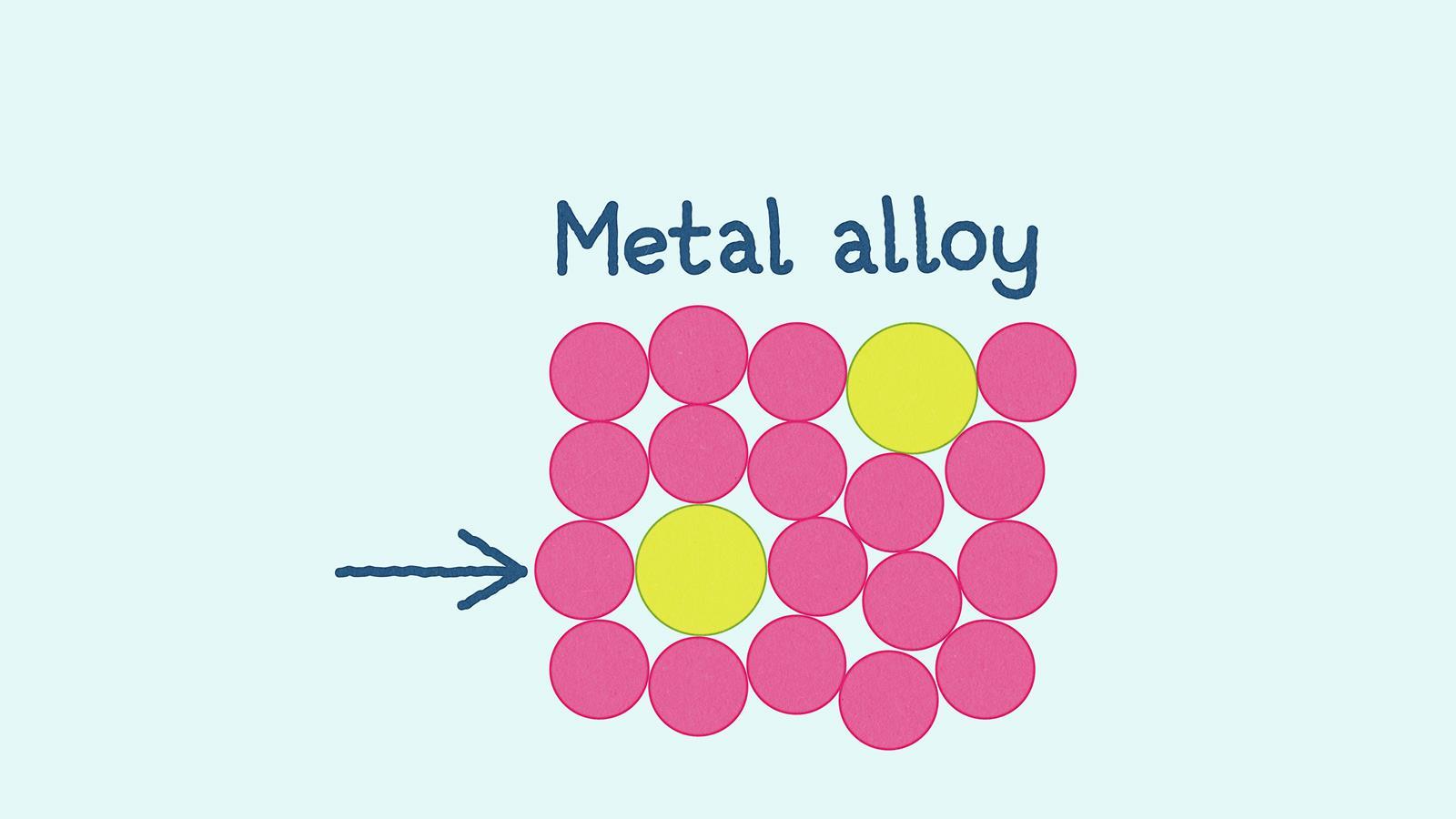
Alloys are mixtures of two or more elements where at least one is a metal. Metallic bonds are the strong electrostatic attractions between the positively charged metal ions and the delocalised electrons
In an alloy, the atoms are different sizes which distorts the layered structure. This means greater force is needed to make the layers slide over one another, which makes an alloy harder and stronger than the pure metal
Did you know …?
Aluminium alloys are used to make aircraft, because they’re lightweight and very strong. They are also corrosion resistant due to the ability of aluminium to form a thin protective layer of aluminium oxide.
All illustrations © Dan Bright
Want more posters on bonding?
Try these posters with classroom-ready activities for 14–16 learners:
Downloads
EiC metallic bonding poster 14–16
PDF, Size 0.27 mbEiC Metallic bonding fact sheet 14-16
PDF, Size 0.11 mbEiC Metallic bonding student worksheet
Handout | PDF, Size 0.21 mbEiC Metallic bonding student support worksheet
Handout | PDF, Size 0.22 mbEiC Metallic bonding teacher notes and answers
Handout | PDF, Size 0.18 mbEiC metallic bonding fact sheet 14–16
Word, Size 0.43 mbEiC Metallic bonding student worksheet
Editable handout | Word, Size 0.48 mbEiC Metallic bonding student support worksheet
Editable handout | Word, Size 0.49 mbEiC Metallic bonding teacher notes and answers
Editable handout | Word, Size 0.44 mb







































No comments yet