From particle diagrams to properties through the phase changes, here’s everything your 11–14 learners need to understand about states of matter
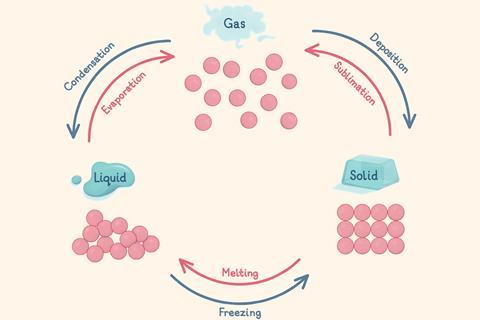
Matter is anything that has mass and takes up space. Everything around us is made of matter and there are three states of matter – solid, liquid and gas. Particle diagrams explain the properties of solids, liquids and gases and they show how the particles are arranged.
View and download more infographics
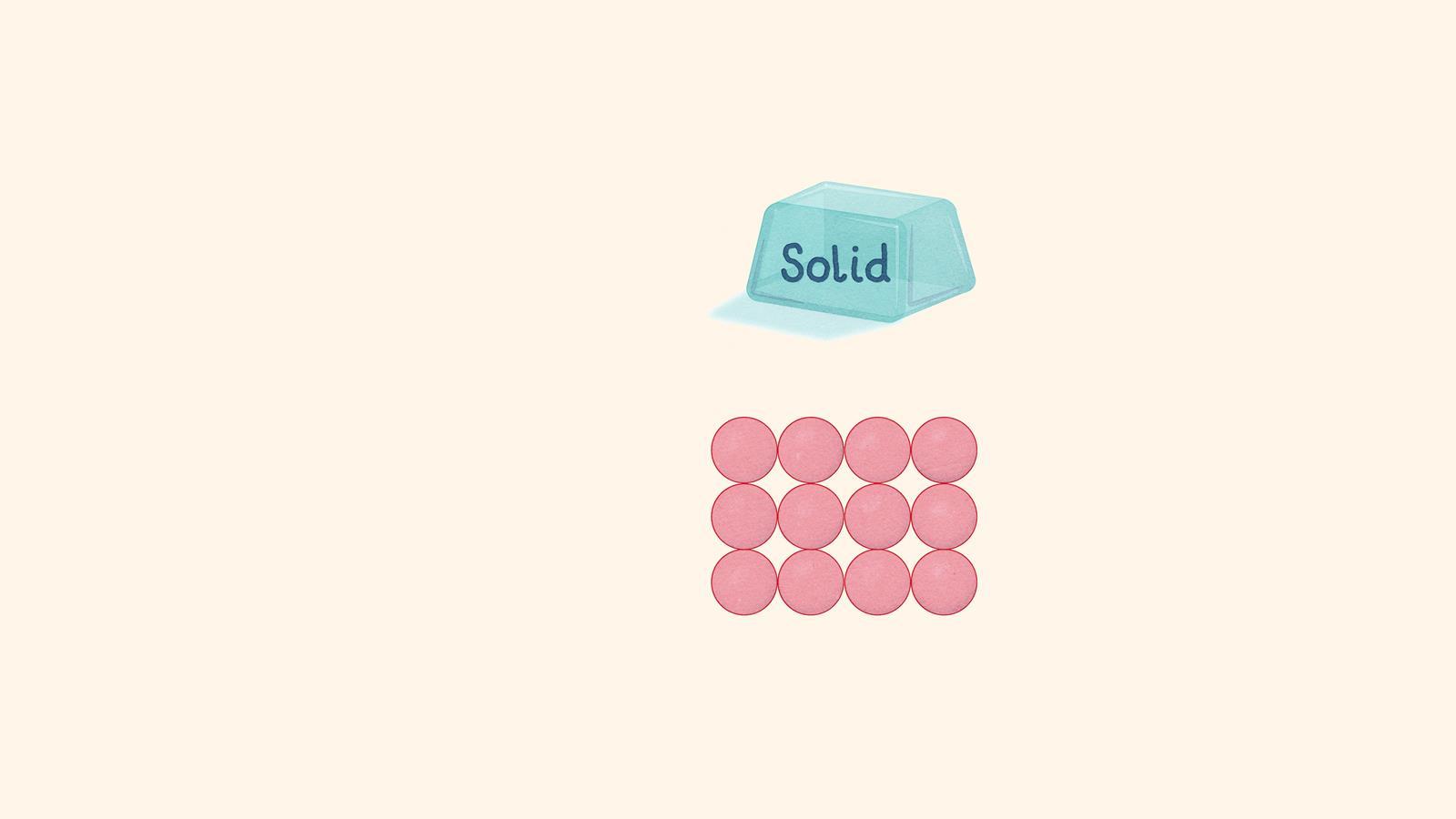
A solid has a defined shape and volume. The particles are touching and vibrating
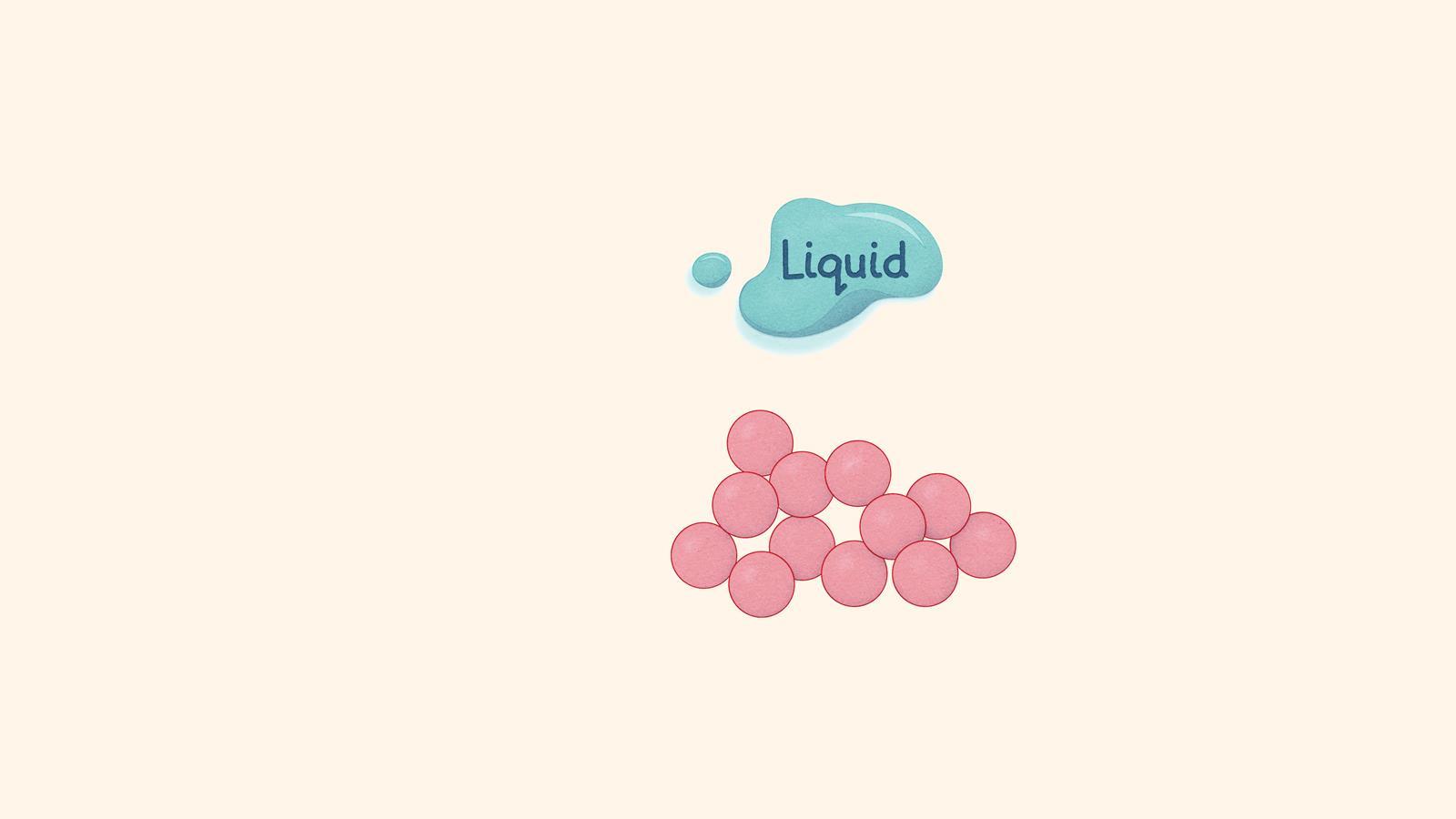
A liquid has a defined volume but not a defined shape. The particles are touching and moving randomly
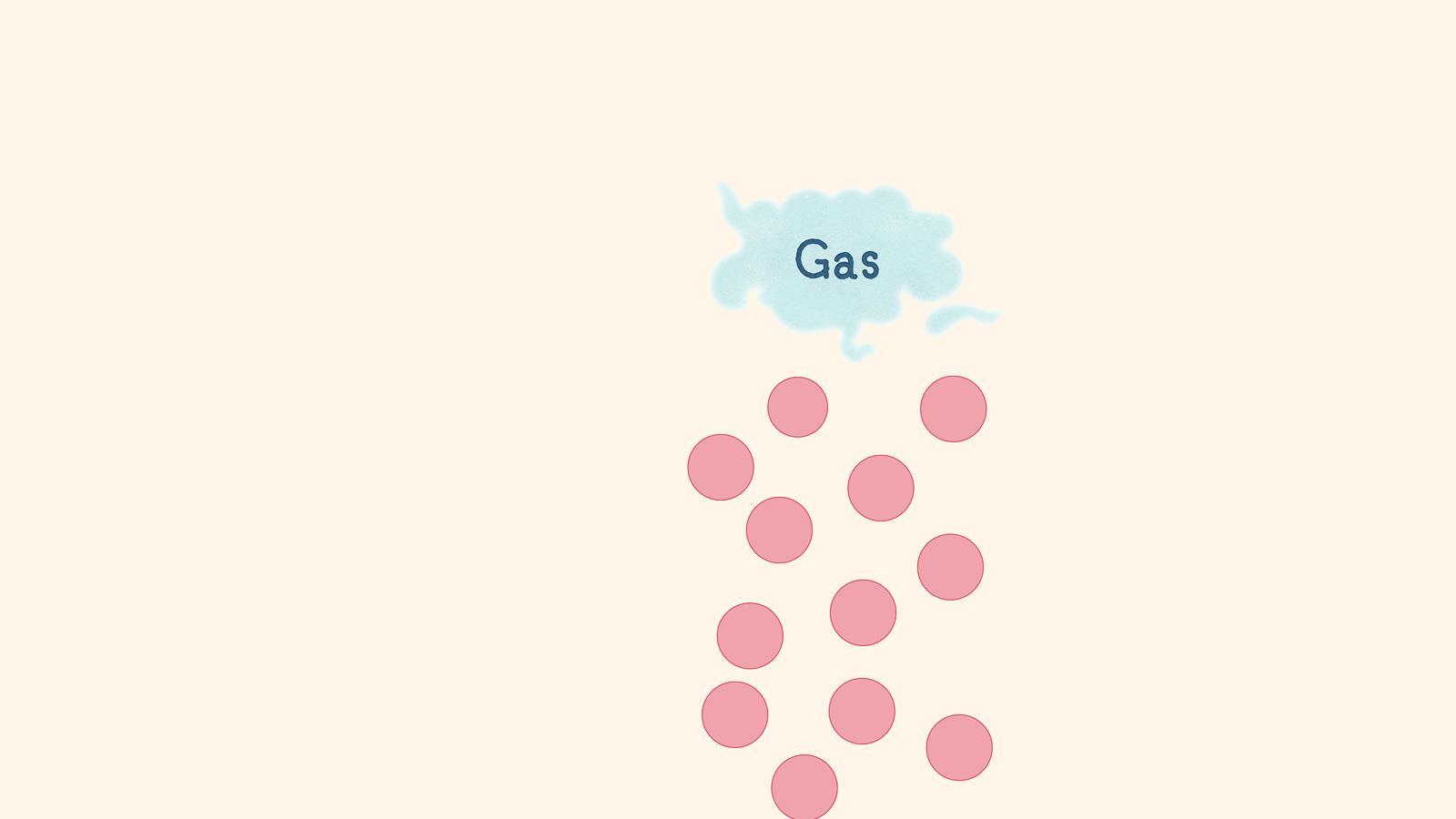
A gas has no defined shape or volume. The particles move randomly and have a large distance between them
Properties of states of matter
| Solid | Liquid | Gas | |
|---|---|---|---|
|
Shape |
fixed |
takes shape of the bottom of the container |
takes shape of container |
|
Volume |
fixed (cannot be compressed) |
fixed (cannot be compressed) |
can be compressed |
|
Particle movement |
can vibrate in place but cannot move out of position |
constantly moving and can slide over each other |
constantly moving |
|
Kinetic energy |
low |
medium |
high |
|
Fluidity |
cannot flow |
can flow |
can flow |
|
Real-world example: making coffee |
ground-up coffee beans |
water in the coffee machine |
when the water is heated in the coffee machine, some of the water becomes a gas as steam |
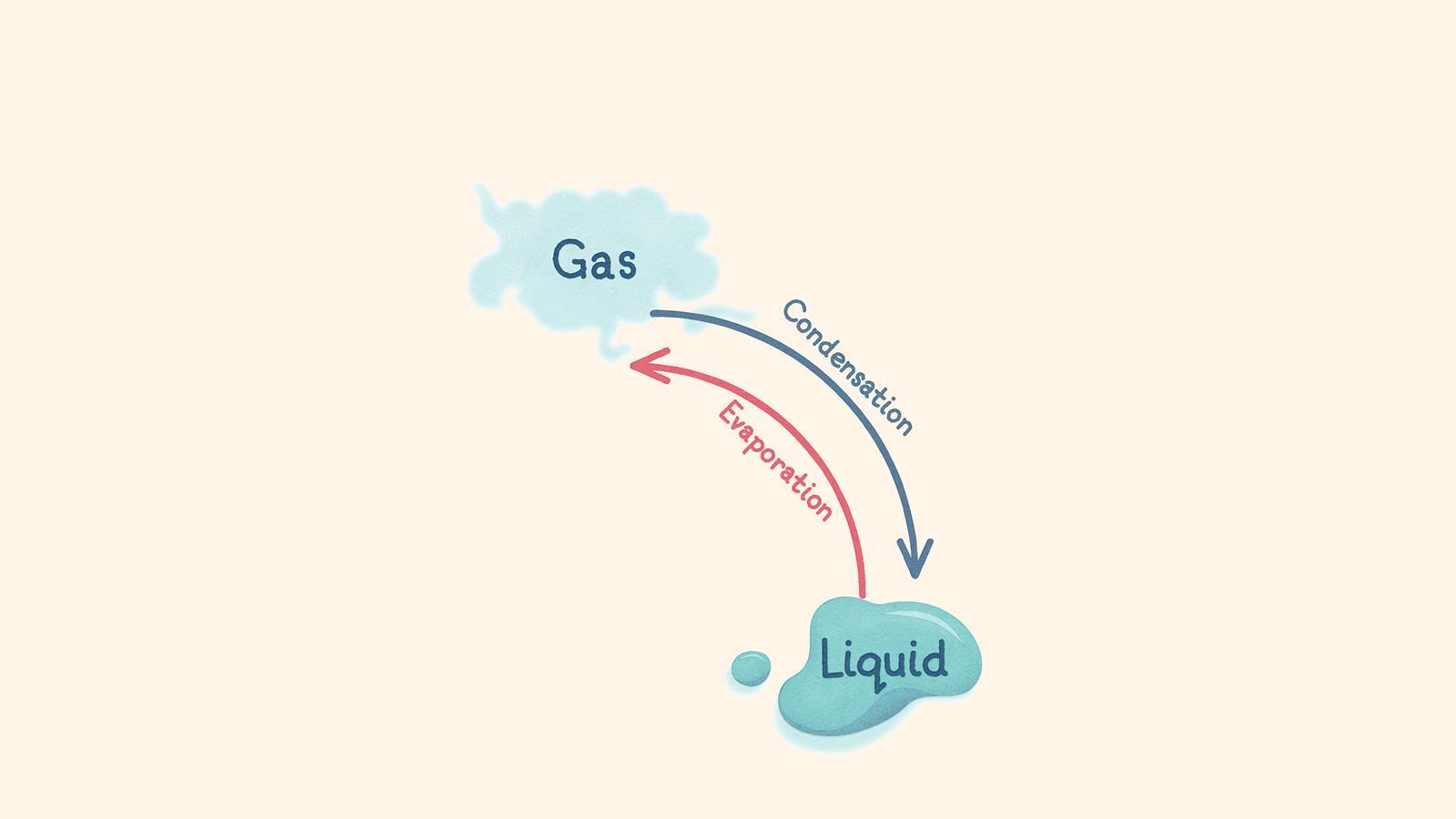
When the temperature of a gas decreases, particles lose energy and turn into a liquid (condensation)
When the temperature of a liquid increases, particles at the surface gain energy and overcome the forces of attraction. The particles turn into a gas (evaporation) and move randomly and freely. This can happen below the boiling point
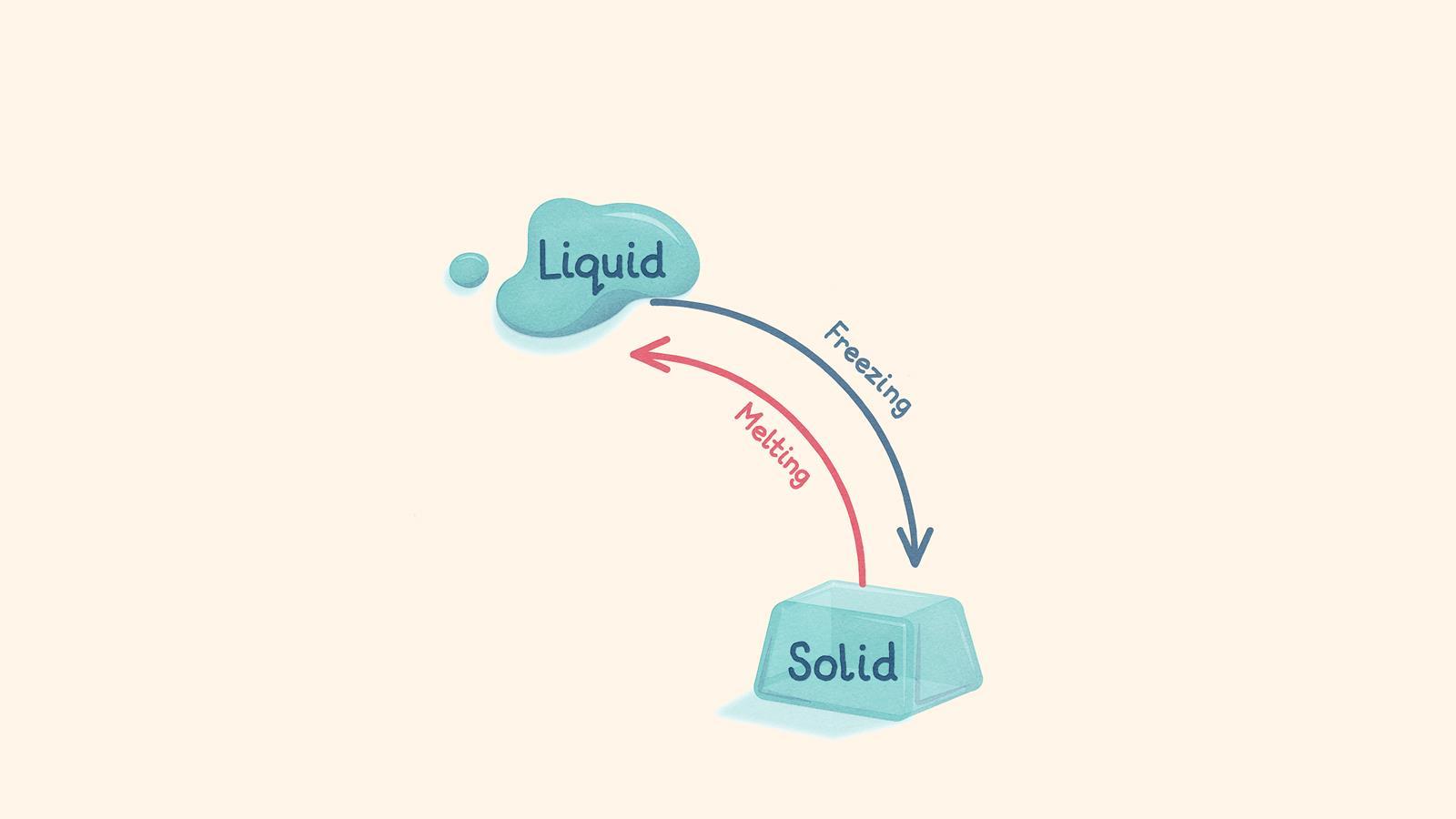
When the temperature of a liquid decreases, it loses energy and turns into a solid (freezing)
When a solid reaches its melting point, it gains energy and turns into a liquid (melting). The forces between particles are overcome and the particles can slide past each other
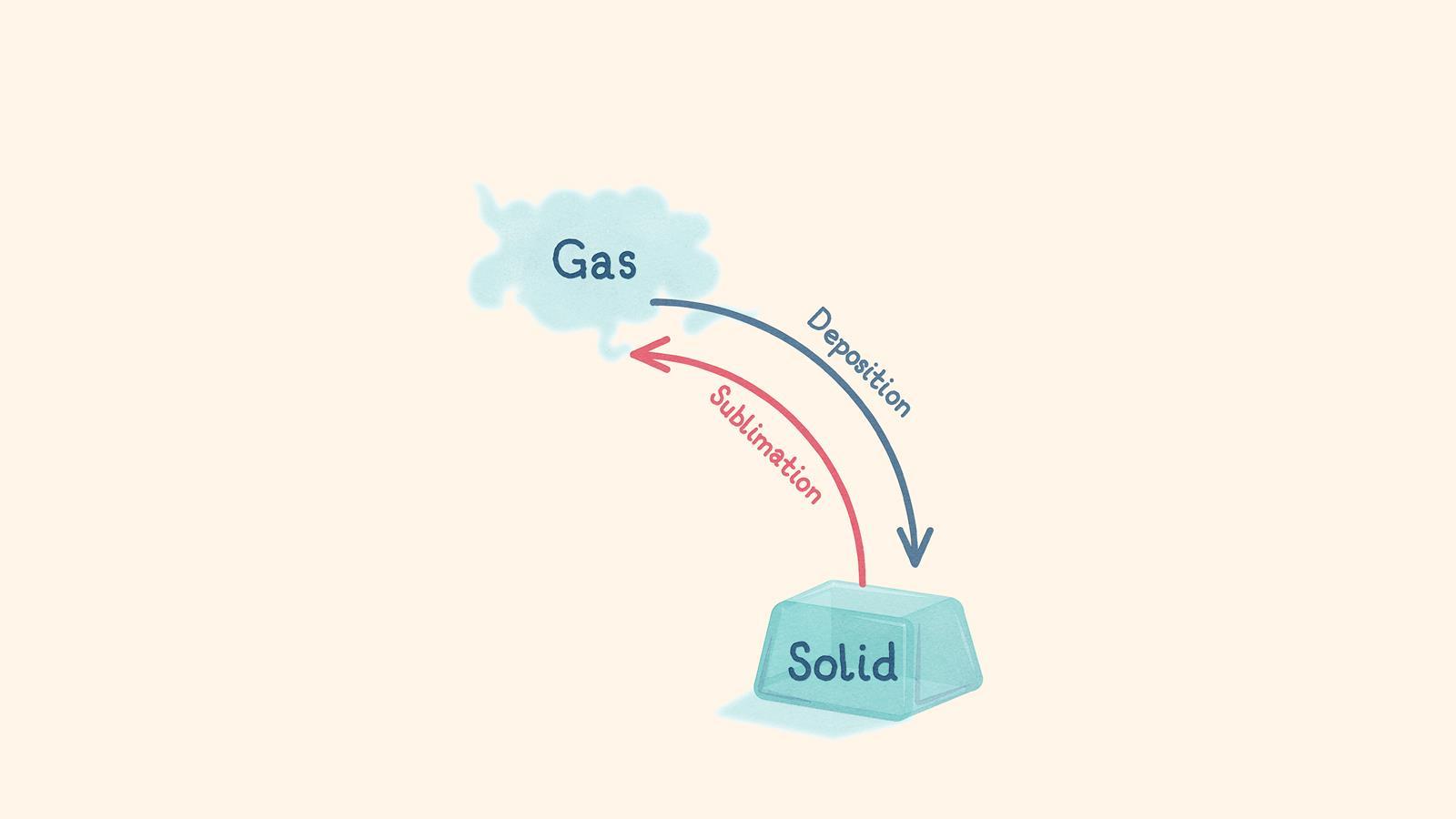
When the temperature of a gas decreases quickly, the particles lose energy and stop moving about. It turns into a solid (deposition)
When a solid is heated quickly, the particles gain energy. It turns into a gas, without first becoming a liquid (sublimation)
Did you know … ?
Water is an example of a compound which is found naturally in all three states of matter – ice as a solid, water as a liquid and water vapour as a gas.
Want more …
… posters for your 11–14 learners?
Downloads
States of matter poster
PDF, Size 0.5 mbStates of matter fact sheet
Handout | PDF, Size 0.13 mbStates of matter fact sheet
Editable handout | Word, Size 0.48 mb




































2 readers' comments