Recreate an early ‘breathalyser’ test to provide context when teaching the oxidation of alcohols
In this experiment, show learners the reaction used in early forms of breathalysers. As air containing ethanol vapour passes through the acidified potassium dichromate solution for several minutes, learners can observe a visible change from the orange of dichromate(VI), through brown, to the green of chromium(III) ions.
This resource accompanies the article How forensic scientists uncover evidence in Education in Chemistry. The article explores the groundbreaking chemical analysis forensic scientists use to fight crime and even improve our health.
Introduction
This is a teacher demonstration which takes under 10 minutes to perform. Use it to provide context when teaching the properties of alcohols. The ‘breathalyser’ reaction is also a good opportunity to discuss the dangers of drinking and driving, although modern breathalysers use electronic methods to detect and measure alcohol concentration.
Find more details on how chemists use infrared spectrometry to detect alcohol in drivers’ breath on page 82 of Modern chemical techniques: infrared spectroscopy. Read the How Stuff Works article for more information about the chemistry of the chemical breathalyser, as well as more modern devices.
Learners will require prior knowledge of redox, oxidation states and properties of transition elements, in addition to the oxidation of alcohols.
Questions 1–7 of the Student worksheet rehearse the essential chemical facts of the reaction. Question 8 gets learners to think about the way the application of the reaction in the breathalyser works. Questions 9–13 lead learners to understand how using [O] in oxidation questions works and how much that simplifies the equations. Find the answers in the Teacher notes.
Learning objectives
- Observe an application of the oxidation of primary alcohols using acidified potassium dichromate solution.
- Recall the colour change of the chromium-containing species in the ‘breathalyser’ reaction and the products of oxidation of primary alcohols.
- Understand that you can use the reaction as an analytical test to identify (primary or secondary) alcohols.
- Write redox equations and review transition elements’ properties.
Adaptive teaching
Questions 1–8 are designed to be accessible to post-16 learners with little support. Questions 9–13 are more challenging and may not be appropriate for all. You can do them as a class so you can give help and check answers as you go through them. To further challenge your learners, set Q5 from the 2005 Olympiad paper.
Equipment
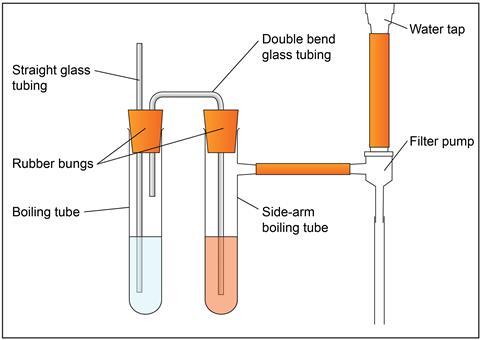
- Measuring cylinder, 25 cm3, x 2
- Boiling tube
- Boiling tube with side arm
- Rubber bung x 2, one with two holes, to fit boiling tubes
- Glass tubing x 2, one straight and one with a double bend (u shape)
- Filter pump attached to the side arm of the boiling tube
- Clamp stand, boss and clamp x 2
- Access to a water tap
- Safety equipment: splashproof goggles and chemical-resistant nitrile gloves
Chemicals
- Ethanol (IDA, industrial denatured alcohol), 95%. Danger: highly flammable liquid and vapour. Harmful if swallowed. May cause damage to organs. See CLEAPSS Hazcard HC040a.
- For the acidified potassium dichromate(VI) solution, 0.1 mol dm-3. Danger: corrosive to skin and eyes. Harmful if swallowed. Respiratory irritant, skin and respiratory sensitiser. Serious health hazard (RE). Serious health hazard (CMR). See CLEAPSS Hazcard HC078c and recipe sheet RB070, or download the Technician notes.
- Potassium dichromate(VI) solid. Danger: May intensify fire; oxidiser. Toxic if swallowed. Harmful in contact with skin. Causes severe skin burns and eye damage. May cause allergic skin reaction. Fatal if inhaled. May cause allergy or asthma symptoms or beathing difficulties if inhaled. May cause cancer or genetic defects. May damager fertility or the unborn child. Causes damage to organs through prolonged or repeated exposure. Very toxic to aquatic life with long-lasting effects.See CLEAPSS Hazcard HC078c.
- Sulfuric acid solution, 1,4 mol dm-3. Warning: causes severe skin burns and eye damage. See CLEAPSS Hazcard HC098a and recipe sheet RB098. You can prepare this solution by diluting a sulfuric acid solution of higher concentration, eg 2.0 mol dm-3.
The reaction also produces:
- Ethanal (acetaldehyde). Danger: extremely flammable. Causes serious eye irritation. May cause respiratory irritation. Suspected of causing genetic effects. May cause cancer. See CLEAPSS Hazcard HC034.
- Ethanoic acid solution (acetic acid). Warning: irritant to skin and eyes. See CLEAPSS Hazcard HC038a.
Health and safety
Read our standard health and safety guidance and carry out a risk assessment before running any live practical. Refer to SSERC/CLEAPSS Hazcards and recipe sheets. Hazard classification may vary depending on supplier.
Wear eye protection throughout. Wear splashproof goggles and chemical-resistant nitrile gloves for preparing and handling the acidified potassium dichromate(VI) solid and solution.
Procedure
-
Wearing chemical-resistant nitrile gloves and eye protection, pour sufficient acidified potassium dichromate solution to submerge the glass delivery tube into the side-arm boiling tube.
-
Pour ethanol into the second boiling tube so that the longer of the two glass tubes is below the surface of the ethanol and the shorter glass tube is not.
-
Attach the side arm of the boiling tube to the pump fitted to the tap.
-
Secure all the equipment using bosses, clamps and stands.
-
Gently turn on the water tap so that air bubbles steadily pass through the ethanol and the acidified potassium dichromate solution.
-
Draw the air containing ethanol vapour slowly through the acidified potassium dichromate solution for several minutes until you can see sufficient green colour. Keep a sample of the unreacted orange acidified potassium dichromate solution next to the side-arm boiling tube to compare the colour change.
-
Turn off the water tap.
More resources
- Watch the Qualitative tests for organic functional groups practical video and use the supporting resources with your classes to identify a set of unlabelled organic compounds, including an alcohol.
- Use this microscale experiment with your learners to investigate the oxidation reactions between acidified dichromate(VI) and primary, secondary and tertiary alcohols.
- Showcase careers with a Forensic scientist’s video job profile. Joni analyses biological samples for the presence of drugs and alcohols in relation to a person’s behaviour or a possible cause of death.
- Read Chemguide’s summary for more of the theory behind the oxidation of alcohols.
- Revisit prior knowledge learners have from their 14–16 studies with two levels of assessment questions on alcohols.
Disposal
- Reuse the ethanol left in the boiling tube, eg as a solvent to remove permanent marker pen from glassware.
- Add the green potassium dichromate solution in sulfuric acid containing ethanal and ethanoic acid to water and pour down a foul-water drain with further dilution. If the solution has not turned green due to the formation of ions, then add solid sodium metabisulfite in small portions with stirring until you obtain a colour change. Mix thoroughly, add some water and pour the solution down a foul-water drain with further dilution.
- Rinse all glassware and wipe up any potassium dichromate solution that might have spilled on surfaces. Do not allow the solution to dry out.
Downloads
The 'breathalyser' reaction student worksheet
Handout | PDF, Size 0.2 mbThe 'breathalyser' reaction teacher notes
Handout | PDF, Size 0.39 mbThe breathalyser reaction technician notes
Handout | PDF, Size 0.23 mbThe 'breathalyser' reaction student worksheet
Editable handout | Word, Size 0.45 mbThe 'breathalyser' reaction teacher notes
Editable handout | Word, Size 0.55 mbThe breathalyser reaction technician notes
Editable handout | Word, Size 0.48 mb
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry
The resource was updated in 2023 with teacher notes and student worksheet by Tim Jolliff and technician notes by Sandrine Bouchelkia.





























No comments yet