Use this poster and fact sheet to boost your students’ understanding of atomic structure
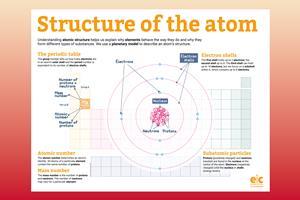
Understanding atomic structure helps us explain why elements behave the way they do and why they form different substances. We use a simple planetary model to describe an atom’s structure, but we will use more complex models at post-16 study.
View and download more infographics
Subatomic particles are protons, neutrons and electrons. Protons (positively charged) and neutrons (neutral) are found in the nucleus at the centre of the atom. Electrons (negatively charged) orbit the nucleus in shells (energy levels)
The first electron shell holds up to two electrons; the second shell holds up to eight electrons. The third shell can hold up to 18 electrons but at this stage, we focus on a subshell within it, which contains up to eight electrons
We can determine some aspects of an element’s atomic structure from its place in the periodic table. The group number tells us how many electrons are in the outer shell (for the first three periods) and the period number is equivalent to the number of electron shells
On the periodic table, you can find an element’s symbol, name and how many subatomic particles it contains. However, it is the atomic number (number of protons) that determines its identity. The number of neutrons and electrons may vary for a given element, but all atoms and ions of a particular element contain the same number of protons
The mass number is the number of protons and neutrons. The number of neutrons may vary for a given element, but the number of protons is always the same
Downloads
Structure of the atom poster
Handout | PDF, Size 0.82 mbStructure of the atom fact sheet
Handout | PDF, Size 0.11 mbStructure of the atom fact sheet
Handout | Word, Size 0.44 mb











































No comments yet