Explore the chemistry and uses of baking powder
The study can be extended to cover all carbonates. Ideally, the latter two stages of this activity should not be done in a laboratory but in a clean environment where students can be allowed to eat the product of their experiment (always very popular).
This usually means that Part 1 has to be done on a separate day.
Equipment required
Part 1
- Test-tubes
- Test-tube holders
- Bunsen burners and mats
- Limewater
- Cobalt chloride paper (Toxic)
- Pipettes
- Baking powder (or NaHCO3 labelled as ‘baking powder’).
- Eye protection.
Part 2
- Sponge cake (see note below).
Part 3
- Sugar
- Golden syrup
- Baking powder
- Baking paper or aluminium foil
- Saucepan
- Wooden spoon
- Cooker or Bunsen burner, tripod, mat and gauze.
Important note: For best results, baking powder must be bicarbonate of soda only. (Some baking powders contain other ingredients too.) In some brands this is ‘baking soda’.
Health and safety
- Wear eye protection.
- Read our standard health and safety guidance
- Cobalt chloride is a carcinogen if inhaled and a skin sensitiser. Minimise handling, eg use forceps and wash hands well after use.
Part 1
This is a simple experiment to show what happens when a hydrogencarbonate is heated. To engage student interest, you could withhold the name of the ‘chemical’ until they have completed the practical work.
Students may find it difficult to believe that something as ‘normal’ as baking powder is a chemical so you may wish to have a labelled laboratory container of sodium hydrogencarbonate ready for them to test as well.
Students may not have come across cobalt chloride paper as a test for water before. If they have not, demonstrate the test at the start of the lesson so they know what they are looking for. If all your paper has gone pink, either put it in a desiccator for a few days or heat some gently in a boiling tube.
It will turn blue again and be ready to use. Bring it to the lesson in a desiccator.
When the baking powder is heated the gas produced can be passed through a delivery tube into limewater in a test-tube. However, there is a danger of suck-back with this method so it may be better for students to use a pipette to collect the gas.
As long as they do not squirt cold liquid into the hot test-tube, this is an easy and safe method. For best results, ensure students use only about 1 cm3 limewater. The limewater should go milky and students should recall that this is a test for CO2.
The cobalt chloride paper should turn pink, which indicates the presence of water. This can be harder to see but some water usually condenses on the inside of the boiling tube and the cobalt chloride paper changes colour if placed over this.
Students should be familiar with word equations but this may be the first symbol equation they have met so they could need some guidance.
Part 2
Show students some cake. Look at the recipe and discuss why baking powder might be included in the list of ingredients. You might wish to prepare a cake without baking powder for comparison (it can be kept in a freezer and re-used over several years).
Part 3
This part of the activity can be done in the laboratory but will be most effective if students have the opportunity to eat the proceeds of their hard work. If they are to do so, the experiment needs to be done in a food technology room or a kitchen.
What to do
Part 1
Set up the equipment shown in the diagram. Heat the baking powder gently.
What can you see on the side of the test-tube and what happens to the cobalt chloride paper when you heat the baking powder? What does the test indicate is present?
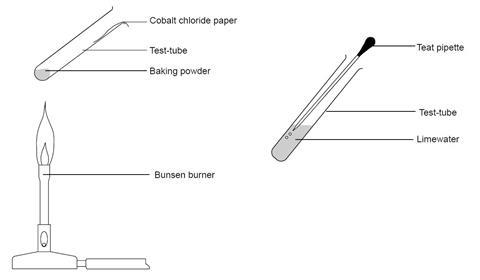
Take a pipette and squeeze all the air out of it. Put it into the test-tube and suck up some of the gas inside. Squirt the gas into the test-tube of limewater.
What do you see? What does this tell you about the gas that has been produced?
The chemical name for baking powder is sodium hydrogencarbonate. You may see it called bicarbonate of soda in the supermarket. This is the old name for the same stuff. It has the chemical formula NaHCO3.

You have found out what two of the products of this reaction are. Put them into the right hand side of the equation.
The other product of this reaction has been put into the equation for you. What do you think it is called? Write the names of all the substances underneath their symbols.
Part 2
Here are the ingredients of a sponge cake:
- 175 g self-raising flour
- Rounded teaspoon baking powder
- 3 eggs
- 175 g sugar
- 175 g butter
- Half teaspoon vanilla extract.
Look at the cake.
What has happened to the baking powder during cooking?
What effect does this have on the cake and why is baking powder included in the recipe?
Part 3
You are going to make some ‘hokey-pokey’ or honeycomb (the stuff in the centre of Crunchie® bars) using baking powder.
You will need
- Sugar
- Golden syrup
- Baking powder
- Baking paper or aluminium foil
- Saucepan
- Wooden spoon
- Cooker or Bunsen burner, tripod, mat and gauze.
What to do
- Put 5 tablespoons of sugar and 2 tablespoons of golden syrup into a saucepan.
- Measure out 1 teaspoon of baking powder and put it on a piece of baking paper or aluminium foil. Keep this separate.
- Heat the sugar and syrup gently over a low heat until the sugar dissolves.
- Keep heating for a further 4 minutes. Keep the heat very low and stir all the time. If you are doing this on a Bunsen burner, you will need to use a tripod and gauze.
- If the mixture boils vigorously, lift the whole pan off the heat and keep stirring so that the mixture does not burn.
- After 4 minutes, remove the pan from the heat, add the baking powder and stir.
- Pour the mixture onto a piece of baking paper or aluminium foil.
What effect does the baking powder have on the syrup mixture?
Why does this happen?
Write a word and a symbol equation for the reaction that takes place when the baking powder gets hot.
Downloads
Baking powder
PDF, Size 0.33 mb
Additional information
This resource is a part of our Inspirational chemistry collection.
Inspirational chemistry book

A collection of resources, aligned with GCSE bodies, to support learners in England, Wales, and N Ireland.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
 Currently
reading
Currently
reading
Baking powder
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35




















































































No comments yet