It’s not easy being green
The competing needs of development and environmental protection are often mentioned in the media and many students have opinions on the subject. Chemistry is seen as a ‘polluter,’ which partly accounts for its poor image among students and the general public.
This activity introduces the concept of atom economy in the context of sustainable development and Green Chemistry. It aims to show that development is necessary but can be achieved in a way which limits environmental damage.
Prior knowledge required
Students need to know/be able to:
- Calculate relative molecular mass (RMM or Mr)
- Know what a reversible reaction is and how the yield of a reaction might be affected by its reversible nature
- Calculate percentage yield – a section on this is included in the resource but it would be better if students had already covered it so that they do not get it mixed up with atom economy.
Further information
Further information on sustainable development is available on various websites, including: http://www.uyseg.org/sustain-ed/Index.htm – this website of the Chemical Industry Education Centre is a good introduction to why development is necessary and how it can be made more sustainable.
Students can calculate their personal sustainability and also how much carbon dioxide they produce in a year. Examples of chemical industries that are going greener are provided, along with links to several other sites.
A quarter of the world’s people have to survive on less than 70p a day. Millions have no health care and the world’s population is expected to increase by about another 3 billion over the next 50 years. Even in developed nations, poverty, education and healthcare could be improved. To help deal with this situation, the world’s economy needs to grow; in particular, the economies of developing nations need to expand. However, economic growth is often linked to environmental pollution problems.
The challenge is to develop in a way that meets the needs of the present generation without compromising the ability of future generations to meet their own needs – in other words, without causing a lot of environmental damage and wasting limited resources. This type of development is called ‘sustainable development’ and it will be more and more critical as the population of the world increases.
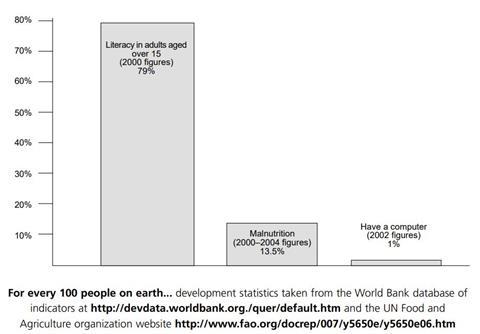
One of the ways in which the chemical industry is working towards sustainable development is by using ‘Green Chemistry.’ One of the basic ideas of Green Chemistry is to prevent pollution and the production of hazardous materials instead of producing them and then cleaning them up.
This means that Green Chemistry:
- Is safe
- Conserves raw materials and energy
- Is more cost-effective than conventional methods.
There are three main ways to make chemical processes ‘greener’:
- Redesign production methods to use different, less hazardous starting materials
- Use milder reaction conditions, better catalysts and less hazardous solvents
- Use production methods with fewer steps and higher atom economy.
Yield
Most of the chemical industry is concerned with turning one material (the raw material) into another one that is more useful and valuable (the product). This process may have several steps and is called a ‘chemical synthesis.’ All the designers of chemical processes want to make the maximum amount of product they can from a given raw material. It is possible to calculate how successful one of these processes is by using the idea of yield.
Atom economy
The idea of yield is useful, but from a Green Chemistry and sustainable development perspective, it is not the full picture. This is because yield is calculated by considering only one reactant and one product. One of the key principles of Green Chemistry is that processes should be designed so that the maximum amount of all the raw materials ends up in the product and a minimum amount of waste is produced.
A reaction can have a high percentage yield but also make a lot of waste product. This kind of reaction has a low atom economy. Both the yield and the atom economy have to be taken into account when designing a green chemical process.
Find out more about chemistry careers
Read about chief executive officer, Daniel who is turning manufacturers’ waste carbon dioxide into chemicals that can be used in everyday products.
Downloads
Green chemistry
PDF, Size 0.25 mb
Additional information
This resource is a part of our Inspirational chemistry collection.
Inspirational chemistry book
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
 Currently reading
Currently readingGreen Chemistry, atom economy and sustainable development
- 33
- 34
- 35




















































No comments yet