Changing minds and surfaces
The key idea is that a short length of –(CF2 )n– polymer can be added to the surface of clothing to change its characteristics.
The polymer is polytetrafluoroethene (also known as a perfluoroalkyl) – the same polymer as that used to make Teflon® coatings. Teflon® and these textile coatings work in the same way. The tightly-bound, non-bonding electron pairs surrounding each fluorine atom are not easily polarised. This prevents the atoms both from hydrogen bonding with water, and from forming dispersion interactions with nonpolar liquids such as oils.
The technology for these coatings was developed by Prof J P Badyal at the University of Durham during the 1990s. The work was done in collaboration with the Ministry of Defence. Staff at the Ministry were interested in producing suits for armed forces personnel that would repel toxic chemicals such as mustard gas (a dispersion that consists of very tiny particles of liquid).
The mechanism of reaction is more complex than that of normal addition polymerisation. The details of the mechanism are still not fully understood and there are a number of different possibilities for how it might occur. This is not covered in the student material.
Prior knowledge required by students
Students will need to have studied addition polymerisation or at least be aware of what polymers are. They should also know something about surfaces – at a minimum, they should be aware that the surface behaves differently from the bulk of a substance. They could perhaps have done the practical activity The surfaces of substances
Suggested lesson plan
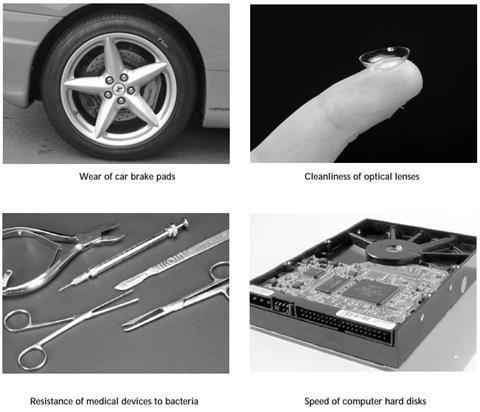
- Begin by showing students the slide entitled What’s the connection? This could be displayed as students are coming into the room. It shows pictures of four seemingly disparate items. The connection between these items is that the properties of each are governed not by what the bulk of the item is made of, but by its surface.
- Demonstrate super-repellant surface properties, either by dropping water onto a super-repellant surface (such as waterproof trousers for hikers), by showing the slide Water repellant surfaces, or by showing the video clip An electrifying way to stay dry available at http://www.research-tv.co.uk/stories/science/plasma/ (accessed November 2005). Alternatively, students could try dropping water onto a variety of surfaces themselves and looking at the shape of the drop formed on the surface. They could try cotton and other fabrics, Teflon® sheets, glass slides and waterproof trousers. (Note that the waterproof trousers on the market are generally not made using the technology featured in this activity and often do not have such convincing water-repellant properties as the fabrics shown on the slide and in the video clip.)
- Students do the first few questions on the student sheet.
- Show the video clip from http://www.research-tv.co.uk/stories/science/plasma/
- If possible do a demonstration with a plasma ball: As the electricity passes through the gas in the ball it excites the gas. You can see this happening because light is produced. By putting your finger on the outside of the ball you can localise the glow on your finger. If a textile is used instead of your finger on the glass surface, then it is possible to coat the textile with the excited gas, which forms a polymer coating.
- Students complete the rest of the student sheet.
- You could conclude with a discussion of surfaces that may be in use in the future.
What’s the connection?
It always happens when you’ve spent ages getting ready to go out or when you’re really trying hard to impress someone: you spill something on your clothes that just will not come out, like Ribena® or Coke. It would be very useful to have clothing and textiles that liquids just rolled off and didn’t stain. Useful too if they did not pick up smells like cigarette smoke or cooking.
Scientists at the University of Durham have been working on a way to achieve just that. Their method involves fixing a short length of a polymer that repels water and other liquids to a fabric. The liquids then just roll off the fabric, leaving it unchanged.
Fabrics that behave like this are called ‘super repellant’. The technology was developed with the Ministry of Defence and its original purpose was to provide protective suits for the armed forces in case of chemical warfare. However, it is now finding its way into many other applications.
The polymer used to make fabric super repellant is the same one that is used to make Teflon® coatings on frying pans. It is called polytetrafluoroethene. A section of the polymer looks like this
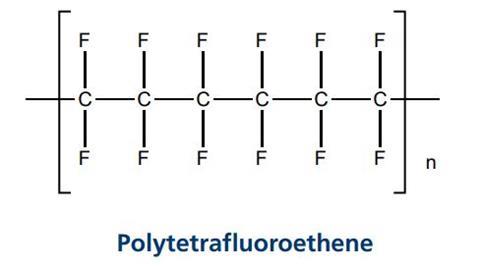
It has been possible to coat textiles with this polymer for many years but the method used was very expensive. The polymerisation reaction was carried out in solvents and then the mixture was sprayed onto the textile. The solvents would evaporate leaving the polymer coating on the fabric. The main problems with this were that a large quantity of solvents was needed and the coating left on the fabric was very thick. Scientists were keen to develop a new method which would be both greener and cheaper.
Put this in context
Watch the video about consumer products technician, Robert who uses polymers to develop materials that improve the properties of products such as chewing gum and cosmetics.
Downloads
Changing the surface
PDF, Size 0.42 mb
Additional information
This resource is a part of our Inspirational chemistry collection.
Inspirational chemistry book

A collection of resources, aligned with GCSE bodies, to support learners in England, Wales, and N Ireland.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
 Currently
reading
Currently
reading
Changing the surface
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35























































































No comments yet