This question sheet would fit into work on rates of reactions and particle size or nanotechnology.
For centuries, silver has been used in jewellery, coins and decoration. It is less well known that silver has been valued for almost as long for its medicinal properties. In Ancient Greece silver was used to purify drinking water, and just decades ago doctors applied a thin layer of silver to large wounds to prevent infection and promote healing.
Silver became less frequently used to prevent infection once antibiotics were invented, but now it is being used again because of the rise in drug-resistant bacteria and new discoveries in materials sciences. The trouble with silver is that the properties that make it useful for jewellery make it less useful in medicine.
Put this in context
Watch the video of nanotoxicologist, Vicki explaining her role in examining how nanomaterials interact with our bodies to ensure they are safe to use or consume.
Questions
- What are the properties of silver that make it useful for jewellery and coins?
- Why would these properties make it less useful for medicine?
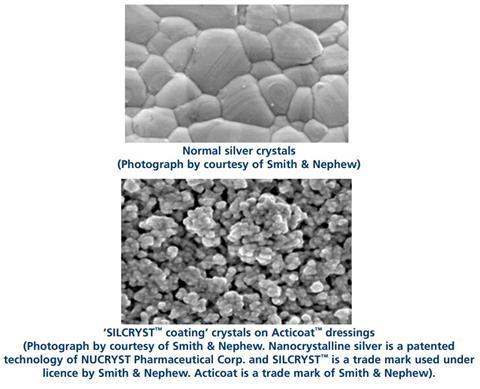
- Look at the pictures of silver crystals. How are the ‘SILCRYST™ coating’ crystals on Acticoat™ dressings different from the normal silver crystals?
- What effect will this difference have on the reactivity of the silver? Why?
Nanoparticles of silver are used in wound dressings for burns and serious injuries. The dressing has three layers. There is an absorbent inner core to keep the wound moist, which helps it to heal. Either side of this core is a net made of polythene, which is coated with nano silver. The polythene net is designed so that it does not stick to the wound.
The silver kills a wide range of bacteria. It is important that it can begin to kill the bacteria as quickly as possible. The tiny nanoparticles of silver dissolve very quickly once they are moistened (for example by blood from the wound) and the silver can get to work straight away.
- Why is it important to begin to kill the bacteria as quickly as possible?
- Why will nanoparticles of silver be more effective against bacteria and work better in a wound dressing than normal silver?
For chronic wounds that can persist for months, such as bedsores and leg ulcers caused by poor circulation, better dressings can prevent serious side effects that might make amputation necessary.
- Why is it important for scientists to keep trying to improve medical items like dressings?
Answers
- The properties of silver that make it useful for jewellery are: it is unreactive, shiny and does not dissolve in water.
- These properties would make silver less useful for medicine because if it does not dissolve it will be hard to get it into the body and if it is unreactive it will not do anything even if it does get to the source of the problem.
- The SILCRYST® nanocrystals on the Acticoat® dressings are much smaller than the normal crystals and their surface is much rougher. This means they have a much larger surface area than the normal crystals.
- This will make the silver more reactive as it is the atoms at the surface that are able to react or dissolve. As there are far more atoms at the surface of the nano silver it is much more reactive than normal silver.
- The bacteria will multiply very rapidly if they are not killed. The conditions in a wound are close to optimum for bacteria and they could double in number every 20 minutes, which would quickly lead to an infection. The infection could then spread elsewhere in the body.
- The nanoparticles are more effective against the bacteria as they dissolve far more quickly than larger particles. Nanosilver can therefore get to work faster than normal silver.
- If dressings are improved, this may mean that fewer people get infections that could become serious and make them very ill or lead to an amputation. Improving simple treatments could help reduce the need for more difficult ones.
Downloads
Nanosilver in medicine
PDF, Size 0.24 mb
Additional information
This resource is a part of our Inspirational chemistry collection.
Inspirational chemistry book

A collection of resources, aligned with GCSE bodies, to support learners in England, Wales, and N Ireland.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
 Currently
reading
Currently
reading
Nanosilver in medicine
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35























































































No comments yet