A three part practical with tense results
The purpose of this activity is to look at the effect that applying the principles of Green Chemistry (see The twelve principles of Green Chemistry) can have on the environmental impact of a well known process: dry cleaning. Students first examine what happens in ‘ordinary’ cleaning and consider a simple explanation of how a detergent works. They then look at dry cleaning and the impact the solvents used in this process can have on the environment. Finally, the ‘greening’ of this process through the use of liquid carbon dioxide as a solvent is discussed.
Background information – surface tension
Water molecules hold on to each other tightly and create a surface tension. In order to use water to clean grease from an item, the surface tension has to be reduced to allow the water to wet the thing you are trying to clean. Surface tension is the force that makes a blob of water stay together and not spread out. It allows pond skaters and other insects to walk across water and also enables a pin to float. If you look closely at drops of water you can see that they try to form spheres. Gravity stretches out drops that cling to an eye dropper or a tap.
However, when the drops fall, they become spherical. The shape of a water drop is a result of surface tension. Water is composed of molecules each consisting of two hydrogen atoms and one oxygen atom. These molecules are attracted to each other. In the middle of a drop of water, each molecule is surrounded by other molecules on all sides so it is pulled equally in all directions by these attractive forces. On the surface, however, the molecules only experience attractive forces in certain directions: across the surface and inward.
This causes the water to try to form a shape with the smallest possible surface area – a sphere. Gravity causes water drops resting on a surface, to flatten out as shown in Figure 1.
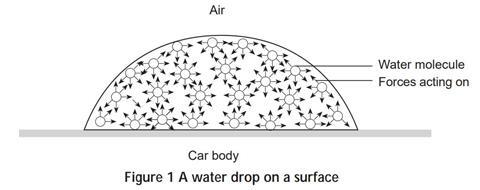
Part 1 Practical work
Equipment required – normal cleaning
- Test-tubes
- Oil – in plastic dropper bottles or with pipettes
- Washing up liquid – in plastic dropper bottles or with pipettes
- Access to water
- Bungs.
Emphasise to students the need to mix the oil and water without shaking so hard that a lot of foam is formed.
Equipment required – how detergents work
- A few clean 2p coins
- Plastic dropping pipettes
- Paper towels
- Washing up liquid
- Solid soap
- Cooking oil
- Paraffin wax
- Sugar
- A soft lead pencil (the ‘lead’ is actually graphite).
Emphasise to students the need to make sure the 2p coin is cleaned properly after each test. If there is any trace of washing up liquid or soap on the coin when another substance is being tested the results could be inconclusive.
What to do
- Fill the test-tubes about 1/4 full with water and pour about 1/2 cm oil on top.
- Put the bung in the top of one of the tubes and mix the oil and water by turning the test-tube upside down a few times. Do not shake it.
- Watch what happens when you leave the mixture to stand.
- Add a few drops of washing up liquid to the other test-tube. Mix the contents in the same way as you did with the first test tube. Do not shake it hard or you will just make lots of foam.
- Watch what happens when you leave the mixture to stand.
- Record your observations carefully.
Questions - part 1
- 1. If you drop oil on your shirt while you are cooking, will water on its own remove it? Why?
- 2. If you put water and washing up liquid on your shirt, will that remove the oil? Why?
Washing up liquid is a type of substance known as a detergent. This experiment has shown that detergents help to break up oil into smaller droplets so that it can mix with water, which makes it easier to get things clean. The next experiment will help you understand how detergents work
What to do next
- Using a pipette, put water onto a 2p coin one drop at a time.
- Count how many drops you can get to balance on the coin.
- Dry the coin carefully and repeat the test twice.
- Do the same experiment again, but this time rub the coin with one of the test substances before you drop water onto it.
- Record your results in a table.
More questions - part 1
- What shape does the water make on the coin just before it falls off? Draw what you saw.
- We use soap and washing up liquid for cleaning. What effect did these substances have on the number of drops you could balance on the coin?
- Oil and wax are substances we might want to remove when we clean something. What effect did these substances have on the number of drops of water you could balance on the coin?
- Explain how adding a detergent to your washing up water makes it easier to clean greasy dishes.
Answers – part 1
- Water alone will not remove the oil because oil and water do not mix.
- Water and washing up liquid may remove the oil because the washing up liquid helps the water and oil to mix. This allows the oil to be washed away in the water.
- The water forms a curved or bulging shape on the top of the coin as shown in Figure 2.
- The washing-up liquid and soap made the number of drops that would fit onto the coin decrease.
- The oils made the number of drops of water that would fit on the coin increase.
- Adding a detergent to the water enables the oil and water to mix together. The oil will be held in the water by the detergent molecules, which act as a bridge between the oil and water molecules. This allows the oil and grease to be washed away in the water and the dishes become clean.
This resource contains part 2 & 3 which look into dry-cleaning and the advantages of using perchloroethene or PERC. Download to find more.
Find out more about chemistry careers
Watch the video about microplastics toxicologist, Stephanie who investigates the potential toxicity of plastic fragments in the air, as well as understanding their impact on human health.
Downloads
Dry cleaning
PDF, Size 0.39 mb
Additional information
This resource is a part of our Inspirational chemistry collection.
Inspirational chemistry book

A collection of resources, aligned with GCSE bodies, to support learners in England, Wales, and N Ireland.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
 Currently
reading
Currently
reading
Dry cleaning and green Chemistry
- 31
- 32
- 33
- 34
- 35























































































No comments yet