Why do feet smell? How do chemists help to provide a solution to the problem?
This activity is based on the science behind the consumer product ‘Purista’, a treatment for textiles which helps to kill the bacteria that grow on clothing and cause it to smell.
The activity requires background knowledge of a number of areas of science:
- Bacteria
- Acids/bases and neutralisation
- Reversible reactions
- Ionic bonding.
Further information about the product is available on the company’s website http://www.purista.co.uk (accessed November 2005) but there is very little scientific information there.
‘Purista’ is the marketing name of the bacteriocide PHMB or Poly(hexamethylene biguanide hydrochloride). It has long been used in swimming pools and contact lens solutions but has now been developed by Arch Chemicals to provide near-permanent antibacterial protection for clothing.
The polymer contains positive groups that appear regularly along its chain:
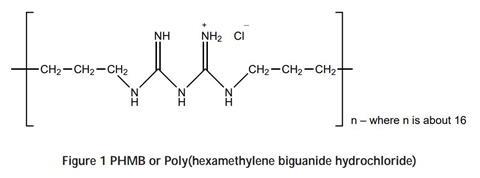
These positive groups can bind to cellulose fibres, such as cotton or viscose. The fibres contain carboxylic acid groups formed by oxidation of alcohol groups during processing of the raw material:
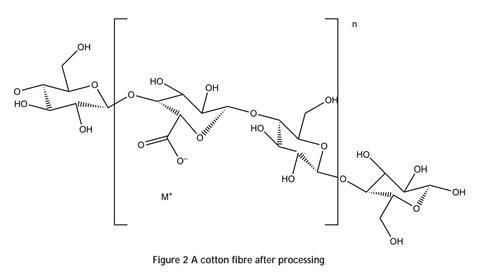
In solutions above about pH 6, the carboxylic acid groups dissociate to give carboxylate ions, which are negatively charged. These carboxylate ions bond to the positive charges on the PHMB molecule and hold it in place. Several bonds form between each PHMB molecule and cotton fibre, which join together a little like Velcro®.
This holds the PHMB in place even during washing. No bacteria have yet been found that are resistant to PHMB, even though it has been used in swimming pools for many years.
Suggested lesson plan
- To introduce the lesson you could show the slide Odd one out? provided in this resource. The bacteria is the odd one out because the rest of the items contain PHMB, sometimes known as ‘Purista®’, which kills bacteria.
- The Belle and Bunty website (http://www.belleandbunty.co.uk, accessed November 2005) sometimes has a video of their latest show, which you could use as an introduction.
- Students work through the information and questions. For greater impact, you could put the pictures of bacteria up on a screen using a data projector. Some sensitivity may be required if you have students in the class who have a body odour problem.
- The material could lead to a discussion of how science is presented to the general public. Why is such a scientific product marketed without any mention of how it works? Can students think of other products where this is also the case?
The problem
Your clothes are next to your skin. Your skin produces oils and sweat, which end up on your clothes. On its own this mixture does not smell bad (in fact, scientists think that sweat contains chemicals called pheromones which help to attract members of the opposite sex).
The smell arises when bacteria are present. Bacteria live on both your skin and your clothes and they thrive in these environments
In low numbers these bacteria are not a problem as they are mostly harmless and unnoticeable.
However, as the bacteria multiply, they produce waste products, and these can be substances which have an unpleasant smell. A bacterial population above about 105 (100 000) per gram of clothing will produce a noticeable smell. A population of 106 bacteria per gram causes a medium smell and above 108 per gram there will be a strong smell.
Look at the picture below of T-shirt fibres:

If you look at the middle of the photograph above, you can see that there seems to be some damage to the fibre. This is not visible to the naked eye. Look at the fibre in close-up:

A colony of bacteria is living on the t-shirt fibre. The bacteria glue themselves to it and form a ‘biofilm.’ This biofilm is very difficult to remove and can remain even after washing.
Modern washing practices have not helped this problem. A few years ago it was common to wash many items on a hot wash (60 °C or above). Now, most clothing is washed on a cool cycle at 40 °C and many new fabrics cannot be washed above this temperature.
Scientists at Arch Chemicals have been working on a solution to this problem for a number of years. They have designed a product that manufacturers can apply to clothing to help prevent bacterial growth. This product is a chemical called PHMB and has been used in swimming pools and contact lens solutions for several years. It kills bacteria by puncturing their cell membranes, causing the contents to leak out. It does not have the same effect on human cells so it is safe to use in contact with the skin.
Once the bacteria are dead they can no longer produce smelly chemicals and the fabrics smell fresh for longer. They still need to be washed, but perhaps not as often.
Put this in context
Find out how senior scientist, Phillip leads a team of researchers who look to improve the performance of household products such as toothpaste and shampoo.
Downloads
Nanotechnology and smelly socks
PDF, Size 1.4 mb
Additional information
This resource is a part of our Inspirational chemistry collection.
Inspirational chemistry book

A collection of resources, aligned with GCSE bodies, to support learners in England, Wales, and N Ireland.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
 Currently
reading
Currently
reading
Nanotechnology and smelly socks
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35























































































No comments yet